
PDF Publication Title:
Text from PDF Page: 011
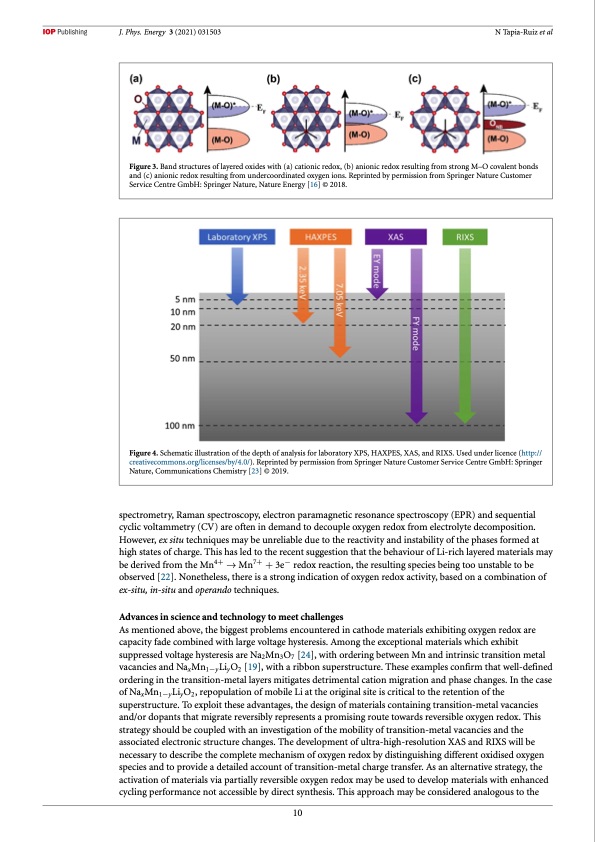
J. Phys. Energy 3 (2021) 031503 N Tapia-Ruiz et al Figure 3. Band structures of layered oxides with (a) cationic redox, (b) anionic redox resulting from strong M–O covalent bonds and (c) anionic redox resulting from undercoordinated oxygen ions. Reprinted by permission from Springer Nature Customer Service Centre GmbH: Springer Nature, Nature Energy [16] © 2018. Figure 4. Schematic illustration of the depth of analysis for laboratory XPS, HAXPES, XAS, and RIXS. Used under licence (http:// creativecommons.org/licenses/by/4.0/). Reprinted by permission from Springer Nature Customer Service Centre GmbH: Springer Nature, Communications Chemistry [23] © 2019. spectrometry, Raman spectroscopy, electron paramagnetic resonance spectroscopy (EPR) and sequential cyclic voltammetry (CV) are often in demand to decouple oxygen redox from electrolyte decomposition. However, ex situ techniques may be unreliable due to the reactivity and instability of the phases formed at high states of charge. This has led to the recent suggestion that the behaviour of Li-rich layered materials may be derived from the Mn4+ → Mn7+ + 3e− redox reaction, the resulting species being too unstable to be observed [22]. Nonetheless, there is a strong indication of oxygen redox activity, based on a combination of ex-situ, in-situ and operando techniques. Advances in science and technology to meet challenges As mentioned above, the biggest problems encountered in cathode materials exhibiting oxygen redox are capacity fade combined with large voltage hysteresis. Among the exceptional materials which exhibit suppressed voltage hysteresis are Na2Mn3O7 [24], with ordering between Mn and intrinsic transition metal vacancies and NaxMn1−yLiyO2 [19], with a ribbon superstructure. These examples confirm that well-defined ordering in the transition-metal layers mitigates detrimental cation migration and phase changes. In the case of NaxMn1−yLiyO2, repopulation of mobile Li at the original site is critical to the retention of the superstructure. To exploit these advantages, the design of materials containing transition-metal vacancies and/or dopants that migrate reversibly represents a promising route towards reversible oxygen redox. This strategy should be coupled with an investigation of the mobility of transition-metal vacancies and the associated electronic structure changes. The development of ultra-high-resolution XAS and RIXS will be necessary to describe the complete mechanism of oxygen redox by distinguishing different oxidised oxygen species and to provide a detailed account of transition-metal charge transfer. As an alternative strategy, the activation of materials via partially reversible oxygen redox may be used to develop materials with enhanced cycling performance not accessible by direct synthesis. This approach may be considered analogous to the 10PDF Image | roadmap for sodium-ion batteries
PDF Search Title:
roadmap for sodium-ion batteriesOriginal File Name Searched:
sodium-ion-batteries.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |