
PDF Publication Title:
Text from PDF Page: 008
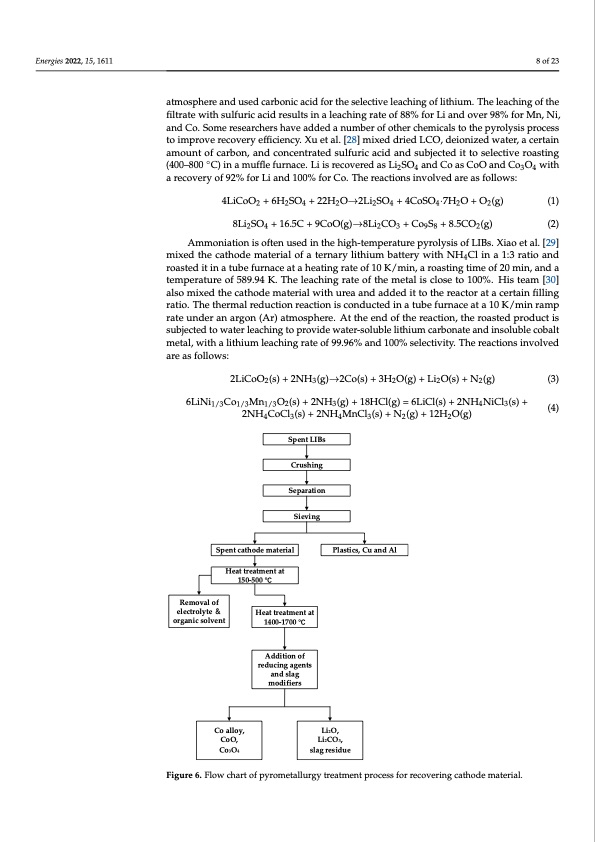
Energies 2022, 15, 1611 8 of 23 Energies 2022, 14, x FOR PEER REVIEW 9 of 24 atmosphere and used carbonic acid for the selective leaching of lithium. The leaching of the filtrate with sulfuric acid results in a leaching rate of 88% for Li and over 98% for Mn, Ni, and Co. Some researchers have added a number of other chemicals to the pyrolysis process to improve recovery efficiency. Xu et al. [28] mixed dried LCO, deionized water, a certain amount of carbon, and concentrated sulfuric acid and subjected it to selective roasting (400–800 ◦C) in a muffle furnace. Li is recovered as Li2SO4 and Co as CoO and Co3O4 with a recovery of 92% for Li and 100% for Co. The reactions involved are as follows: 4LiCoO2 + 6H2SO4 + 22H2O→2Li2SO4 + 4CoSO4·7H2O + O2(g) (1) 8Li2SO4 + 16.5C + 9CoO(g)→8Li2CO3 + Co9S8 + 8.5CO2(g) (2) Ammoniation is often used in the high-temperature pyrolysis of LIBs. Xiao et al. [29] mixed the cathode material of a ternary lithium battery with NH4Cl in a 1:3 ratio and roasted it in a tube furnace at a heating rate of 10 K/min, a roasting time of 20 min, and a temperature of 589.94 K. The leaching rate of the metal is close to 100%. His team [30] also mixed the cathode material with urea and added it to the reactor at a certain filling ratio. The thermal reduction reaction is conducted in a tube furnace at a 10 K/min ramp rate under an argon (Ar) atmosphere. At the end of the reaction, the roasted product is subjected to water leaching to provide water-soluble lithium carbonate and insoluble cobalt metal, with a lithium leaching rate of 99.96% and 100% selectivity. The reactions involved are as follows: 2LiCoO2(s) + 2NH3(g)→2Co(s) + 3H2O(g) + Li2O(s) + N2(g) 6LiNi1/3Co1/3Mn1/3O2(s) + 2NH3(g) + 18HCl(g) = 6LiCl(s) + 2NH4NiCl3(s) + (3) 8Li2SO4 + 16.5C + 9CoO(g)→8Li2CO3 + Co9S8 + 8.5CO2(g) (2) (4) 2NH4CoCl3(s) + 2NH4MnCl3(s) + N2(g) + 12H2O(g) Spent LIBs Crushing Separation Sieving Spent cathode material Plastics, Cu and Al Heat treatment at 150-500 °C Removal of electrolyte & organic solvent Heat treatment at 1400-1700 °C Addition of reducing agents and slag modifiers Active Ingredients Figure 6. Flow chart of pyrometallurgy treatment process for recovering cathode material. Figure 6. Flow chart of pyrometallurgy treatment process for recovering cathode material. Table 2. Several main pyrometallurgy processes. Recycling and Parameter Conditions Leach Efficiency (%) Ref. Disposal Methods Li Co Mn Ni Co alloy, CoO, Co3O4 LCO to H2SO4 ratio 2:1, 20% carbon Li2O, Li2CO3, slag residuePDF Image | Recycling of Lithium Batteries
PDF Search Title:
Recycling of Lithium BatteriesOriginal File Name Searched:
energies-15-01611.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |