
PDF Publication Title:
Text from PDF Page: 009
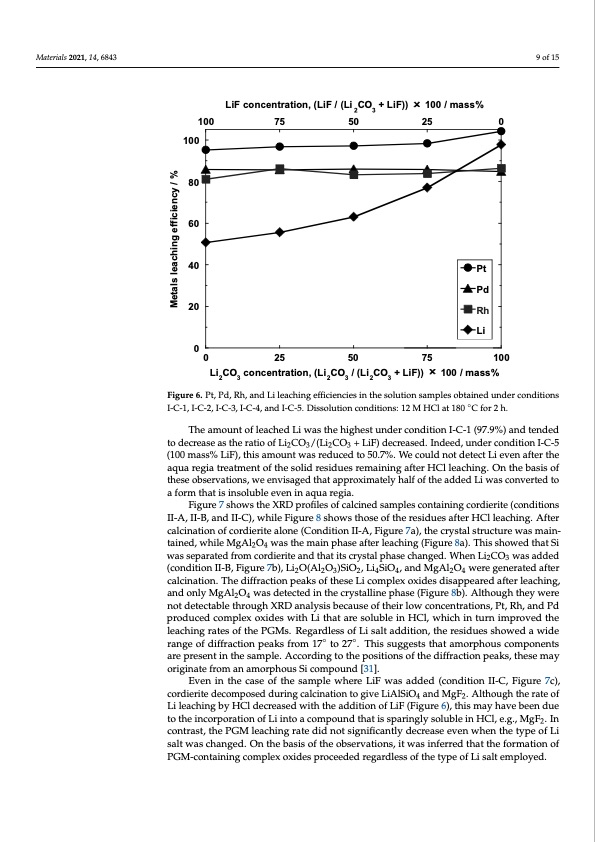
Materials 2021, 14, 6843 Materials 2021, 14, x FOR PEER REVIEW 9 of 15 10 of 16 LiF concentration, (LiF / (Li 2CO3 + LiF)) × 100 / mass% 100 75 50 25 0 100 80 60 40 20 0 Pt Pd Rh Li Li 0 25 50 75 100 Li2CO3 concentration, (Li2CO3 / (Li2CO3 + LiF)) × 100 / mass% Figure 6. Pt, Pd, Rh, and Li leaching efficiencies in the solution samples obtained under conditions Figure 6. Pt, Pd, Rh, and Li leaching efficiencies in the solution samples obtained under conditions ◦ I-C-1, I-C-2, I-C-3, I-C-4, and I-C-5. Dissolution conditions: 12 M HCl at 180 °C ffor 2 h.. The amount of leached Li was the highest under condition I-C-1 (97.9%) and tended The amount of leached Li was the highest under condition I-C-1 (97.9%) and tended to decrease as the ratio of Li CO /(Li CO + LiF) decreased. Indeed, under condition I-C-5 2323 to decrease as the ratio of Li2CO3/(Li2CO3 + LiF) decreased. Indeed, under condition I-C-5 (100 mass% LiF), this amount was reduced to 50.7%. We could not detect Li even after the (100 mass% LiF), this amount was reduced to 50.7%. We could not detect Li even after the aqua regia treatment of the solid residues remaining after HCl leaching. On the basis of aqua regia treatment of the solid residues remaining after HCl leaching. On the basis of these observations, we envisaged that approximately half of the added Li was converted to these observations, we envisaged that approximately half of the added Li was converted a form that is insoluble even in aqua regia. to a form that is insoluble even in aqua regia. Figure 7 shows the XRD profiles of calcined samples containing cordierite (conditions Figure 7 shows the XRD profiles of calcined samples containing cordierite (conditions II-A, II-B, and II-C), while Figure 8 shows those of the residues after HCl leaching. After II-A, II-B, and II-C), while Figure 8 shows those of the residues after HCl leaching. After calcination of cordierite alone (Condition II-A, Figure 7a), the crystal structure was main- calcination of cordierite alone (Condition II-A, Figure 7a), the crystal structure was main- tained, while MgAl2O4 was the main phase after leaching (Figure 8a). This showed that Si tained, while MgAl2O4 was the main phase after leaching (Figure 8a). This showed that Si was separated from cordierite and that its crystal phase changed. When Li2CO3 was added was separated from cordierite and that its crystal phase changed. When Li2CO3 was added (condition II-B, Figure 7b), Li2O(Al2O3)SiO2, Li4SiO4, and MgAl2O4 were generated after (condition II-B, Figure 7b), Li2O(Al2O3)SiO2, Li4SiO4, and MgAl2O4 were generated after calcination. The diffraction peaks of these Li complex oxides disappeared after leaching, calcination. The diffraction peaks of these Li complex oxides disappeared after leaching, and only MgAl2O4 was detected in the crystalline phase (Figure 8b). Although they were and only MgAl2O4 was detected in the crystalline phase (Figure 8b). Although they were not detectable through XRD analysis because of their low concentrations, Pt, Rh, and Pd not detectable through XRD analysis because of their low concentrations, Pt, Rh, and Pd produced complex oxides with Li that are soluble in HCl, which in turn improved the produced complex oxides with Li that are soluble in HCl, which in turn improved the leaching rates of the PGMs. Regardless of Li salt addition, the residues showed a wide leaching rates of the PGMs. Regardless of Li salt addition, the residues showed a wide range of diffraction peaks from 17◦ to 27◦. This suggests that amorphous components range of diffraction peaks from 17° to 27°. This suggests that amorphous components are are present in the sample. According to the positions of the diffraction peaks, these may present in the sample. According to the positions of the diffraction peaks, these may orig- originate from an amorphous Si compound [31]. inate from an amorphous Si compound [31]. Even in the case of the sample where LiF was added (condition II-C, Figure 7c), cordierite decomposed during calcination to give LiAlSiO4 and MgF2. Although the rate of Li leaching by HCl decreased with the addition of LiF (Figure 6), this may have been due to the incorporation of Li into a compound that is sparingly soluble in HCl, e.g., MgF2. In contrast, the PGM leaching rate did not significantly decrease even when the type of Li salt was changed. On the basis of the observations, it was inferred that the formation of PGM-containing complex oxides proceeded regardless of the type of Li salt employed. Metals leaching efficiency / %PDF Image | Recovery of Platinum Group Metals from Auto Catalysts
PDF Search Title:
Recovery of Platinum Group Metals from Auto CatalystsOriginal File Name Searched:
materials-14-06843-v2.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |