
PDF Publication Title:
Text from PDF Page: 006
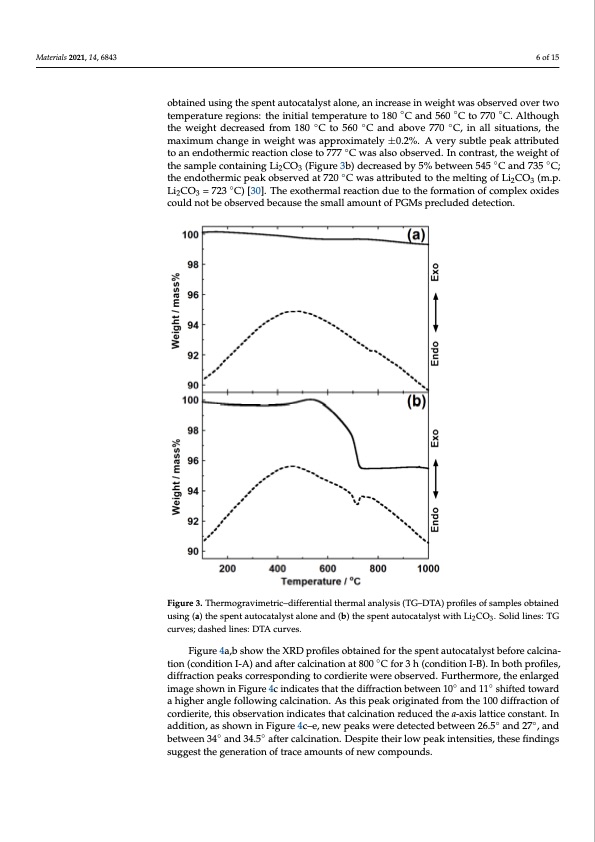
Materials 2021, 14, 6843 6 of 15 obtained using the spent autocatalyst alone, an increase in weight was observed over two temperature regions: the initial temperature to 180 ◦C and 560 ◦C to 770 ◦C. Although the weight decreased from 180 ◦C to 560 ◦C and above 770 ◦C, in all situations, the maximum change in weight was approximately ±0.2%. A very subtle peak attributed to an endothermic reaction close to 777 ◦C was also observed. In contrast, the weight of the sample containing Li2CO3 (Figure 3b) decreased by 5% between 545 ◦C and 735 ◦C; the endothermic peak observed at 720 ◦C was attributed to the melting of Li2CO3 (m.p. Materials 2021, 14, x FOR PEER REVIEW ◦ 7 of 16 Li2CO3 = 723 C) [30]. The exothermal reaction due to the formation of complex oxides could not be observed because the small amount of PGMs precluded detection. Fiigurree33..TThheermrmooggrarvaivmimeterticri–cd–idffiefrfenretinatliathletrhmeraml anlalnyasilsys(TisG(–TDGT–AD)TpAro)fiplerosfoilfesaomfpslaems opbletasinoebdtained usiing(a(a))ththeespsepnetnatuatuotcoactaltyaslyt satloanloenaendan(bd)(tbh)etshpenstpaeuntoacuataolcyastawlyistthwLithCOLi2.CSOol3i.dSloinlieds:liTnGes: TG 23 tion (condition I-A) and after calcination at 800 ◦C for 3 h (condition I-B). In both profiles, tion (condition I-A) and after calcination at 800 °C for 3 h (condition I-B). In both profiles, curves; dashed lines: DTA curves. curves; dashed lines: DTA curves. Figure 4a,b show the XRD profiles obtained for the spent autocatalyst before calcina- Figure 4a,b show the XRD profiles obtained for the spent autocatalyst before calcina- diffraction peaks corresponding to cordierite were observed. Furthermore, the enlarged diffraction peaks corresponding to cordierite were observed. Furthermore, the enlarged image shown in Figure 4c indicates that the diffraction between 10◦ and 11◦ shifted toward image shown in Figure 4c indicates that the diffraction between 10° and 11° shifted toward a higher angle following calcination. As this peak originated from the 100 diffraction of a higher angle following calcination. As this peak originated from the 100 diffraction of cordierite, this observation indicates that calcination reduced the a-axis lattice constant. In cordierite, this observation indicates that calcination reduced the a-axis lattice constant. In addition, as shown in Figure 4c–e, new peaks were detected between 26.5◦ and 27◦, and addition, as shown in Figure 4c–e, new peaks were detected between 26.5° and 27°, and between 34◦ and 34.5◦ after calcination. Despite their low peak intensities, these findings between 34° and 34.5° after calcination. Despite their low peak intensities, these findings suggest the generation of trace amounts of new compounds. suggest the generation of trace amounts of new compounds.PDF Image | Recovery of Platinum Group Metals from Auto Catalysts
PDF Search Title:
Recovery of Platinum Group Metals from Auto CatalystsOriginal File Name Searched:
materials-14-06843-v2.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |