
PDF Publication Title:
Text from PDF Page: 013
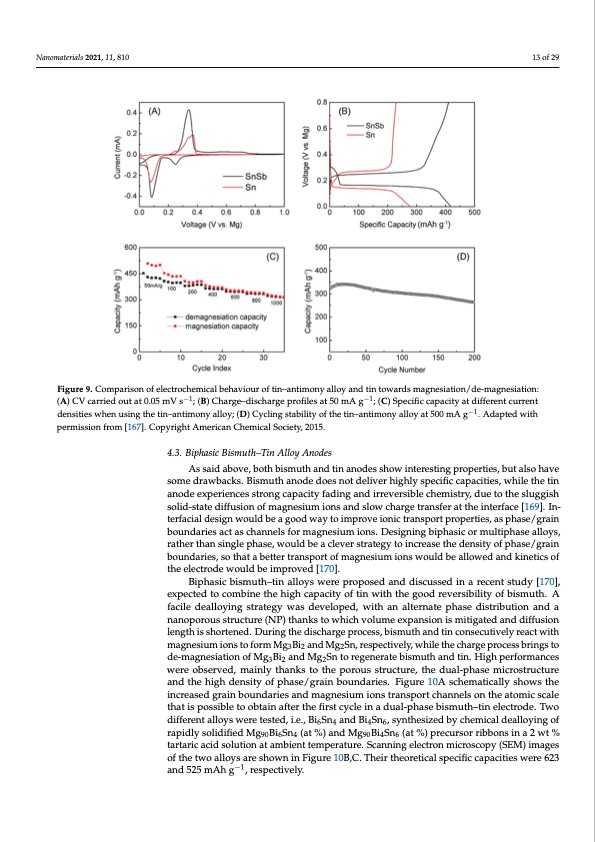
result in lower overpotentials (Figure 9A); (2) improved specific capacity at the same cur- rent density (420 mAh g−1 vs. less than 300 mAh g−1 for pure tin at a 50 mA g−1) (Figure 9B); (3) excellent rate capability with 70% capacity retention (300 mA g−1 at very high cur- Nanomaterials 2021, 11, 810 rent densities of 1000 mA g−1 (Figure 9C); (4) good cyclability, with 270 mAh g−1 after 200 13 of 29 cycles at a current density of 500 mA g−1 (Figure 9D). Figure9.ComFpiagruisroen9.oCfoelmecptraorcishoenmoicfaelebcethroacvhioeumriocafltibne–haanvtimouornoyfatlilno–yanantidmtoinytoawllaorydasnmdatginetsoiwataiordns/dmea-mgnaeg-nesiation: −1 −1 siation/de-magnesiation: (A) CV carried out at 0.05 mV s−1; (B) Charge–discharge profiles at 50 mA (A) CV carried out at 0.05 mV s ; (B) Charge–discharge profiles at 50 mA g ; (C) Specific capacity at different current g−1; (C) Specific capacity at different current densities when using the tin–antimony alloy; −(D1 ) Cy- densities when using the tin–antimony alloy; (D) Cycling stability of the tin–antimony alloy at 500 mA g . Adapted with cling stability of the tin–antimony alloy at 500 mA g−1. Adapted with permission from [167]. Copy- permission from [167]. Copyright American Chemical Society, 2015. right American Chemical Society, 2015. 4.3. Biphasic Bismuth–Tin Alloy Anodes Wang et al. used density functional theory (DFT) calculations to study magnesium As said above, both bismuth and tin anodes show interesting properties, but also have cation diffusion properties in β- and α-Sn. They found a diffusion barrier for an isolated some drawbacks. Bismuth anode does not deliver highly specific capacities, while the tin magnesium atom of 0.395 eV in the α-Sn and of 0.435 eV in the β-Sn. Moreover, a higher anode experiences strong capacity fading and irreversible chemistry, due to the sluggish magnesium concentration decreased the diffusion barrier in the case of α-Sn, while an solid-state diffusion of magnesium ions and slow charge transfer at the interface [169]. In- opposite behaviour was expected for β-Sn. Thus, the α form of tin seemed to represent a terfacial design would be a good way to improve ionic transport properties, as phase/grain better alternative than the β phase as an anode material for MIBs [168]. boundaries act as channels for magnesium ions. Designing biphasic or multiphase alloys, rather than single phase, would be a clever strategy to increase the density of phase/grain 4.3. Biphasic Bismuth–Tin Alloy Anodes boundaries, so that a better transport of magnesium ions would be allowed and kinetics of As said above, both bismuth and tin anodes show interesting properties, but also the electrode would be improved [170]. have some drawbacks. Bismuth anode does not deliver highly specific capacities, while Biphasic bismuth–tin alloys were proposed and discussed in a recent study [170], the tin anode experiences strong capacity fading and irreversible chemistry, due to the expected to combine the high capacity of tin with the good reversibility of bismuth. A sluggish solid-state diffusion of magnesium ions and slow charge transfer at the interface facile dealloying strategy was developed, with an alternate phase distribution and a [169]. Interfacial design would be a good way to improve ionic transport properties, as nanoporous structure (NP) thanks to which volume expansion is mitigated and diffusion phase/grain boundaries act as channels for magnesium ions. Designing biphasic or mul- length is shortened. During the discharge process, bismuth and tin consecutively react with tiphase alloys, rather than single phase, would be a clever strategy to increase the density magnesium ions to form Mg3Bi2 and Mg2Sn, respectively, while the charge process brings to of phase/grain boundaries, so that a better transport of magnesium ions would be allowed de-magnesiation of Mg3Bi2 and Mg2Sn to regenerate bismuth and tin. High performances and kinetics of the electrode would be improved [170]. were observed, mainly thanks to the porous structure, the dual-phase microstructure Biphasic bismuth–tin alloys were proposed and discussed in a recent study [170], and the high density of phase/grain boundaries. Figure 10A schematically shows the expected to combine the high capacity of tin with the good reversibility of bismuth. A increased grain boundaries and magnesium ions transport channels on the atomic scale that is possible to obtain after the first cycle in a dual-phase bismuth–tin electrode. Two different alloys were tested, i.e., Bi6Sn4 and Bi4Sn6, synthesized by chemical dealloying of rapidly solidified Mg90Bi6Sn4 (at %) and Mg90Bi4Sn6 (at %) precursor ribbons in a 2 wt % tartaric acid solution at ambient temperature. Scanning electron microscopy (SEM) images of the two alloys are shown in Figure 10B,C. Their theoretical specific capacities were 623 and 525 mAh g−1, respectively.PDF Image | Overview on Anodes for Magnesium Batteries
PDF Search Title:
Overview on Anodes for Magnesium BatteriesOriginal File Name Searched:
nanomaterials-11-00810.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |