
PDF Publication Title:
Text from PDF Page: 011
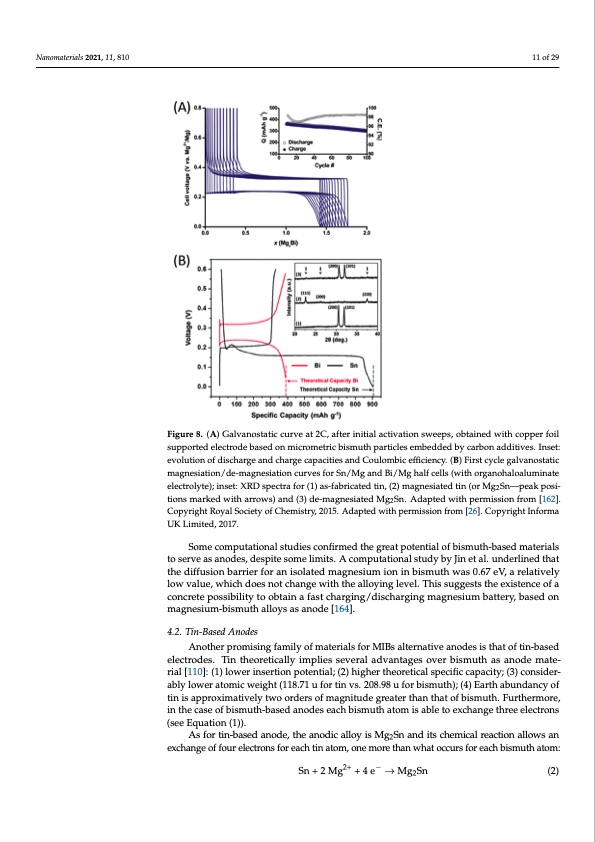
Nanomaterials 2021, 11, 810 pared by ball-milling delivered specific capacity of 300 mAh g−1 at a discharge rate of 2C, with Coulombic efficiency of 98.5% after 50 cycles (Figure 8A). Moreover, a full cell com- posed by a Mg3Bi2 anode and a Mo6S8 cathode in a conventional electrolyte solution of Mg(TFSI)2 0.5 M in dyglime was developed. The full cell showed a voltage profile with a discharge plateau at around 0.6 V. Both the intercalation process on the cathodic si1d1eofa2n9d the de-alloying process of the anode during discharge were corroborated through ex situ XRD measurements. However, full de-magnesiation of Mg3Bi2 was not achieved. Figure 8. (A) Galvanostatic curve at 2C, after initial activation sweeps, obtained with copper foil Figure 8. (A) Galvanostatic curve at 2C, after initial activation sweeps, obtained with copper foil supported electrode based on micrometric bismuth particles embedded by carbon additives. Inset: supported electrode based on micrometric bismuth particles embedded by carbon additives. Inset: evolution of discharge and charge capacities and Coulombic efficiency. (B) First cycle galvanos- evolution of discharge and charge capacities and Coulombic efficiency. (B) First cycle galvanostatic tatic magnesiation/de-magnesiation curves for Sn/Mg and Bi/Mg half cells (with organohaloalumi- magnesiation/de-magnesiation curves for Sn/Mg and Bi/Mg half cells (with organohaloaluminate nate electrolyte); inset: XRD spectra for (1) as-fabricated tin, (2) magnesiated tin (or Mg2Sn—peak electrolyte); inset: XRD spectra for (1) as-fabricated tin, (2) magnesiated tin (or Mg2Sn—peak posi- positions marked with arrows) and (3) de-magnesiated Mg2Sn. Adapted with permission from tions marked with arrows) and (3) de-magnesiated Mg Sn. Adapted with permission from [162]. [162]. Copyright Royal Society of Chemistry, 2015. Ada2pted with permission from [26]. Informa Copyright Royal Society of Chemistry, 2015. Adapted with permission from [26]. Copyright Informa UK Limited, 2017. UK Limited, 2017. Moreover, by 25Mg nuclear magnetic resonance spectroscopy, aimed at understand- Some computational studies confirmed the great potential of bismuth-based materials ing the mechanism and diffusion pathway for magnesium ions in the bismuth anode to serve as anodes, despite some limits. A computational study by Jin et al. underlined that [163], two-phase alloying reactions of magnesium and bismuth were demonstrated, and the diffusion barrier for an isolated magnesium ion in bismuth was 0.67 eV, a relatively such spectroscopy studies enlightened a fast exchange between the two magnesium sites low value, which does not change with the alloying level. This suggests the existence of a in the Mg3Bi2 alloy. concrete possibility to obtain a fast charging/discharging magnesium battery, based on Di Leo et al. proposed the synthesis of bismuth/carbon nanotubes (CNTs) composite magnesium-bismuth alloys as anode [164]. [159]. Electrochemical deposition of bismuth on CNTs from aqueous solution of Bi(NO3)3 was adopted to obtain the composite material. They observed a specific capacity of 180 4.2. Tin-Based Anodes mAh g−1 through CV at a rate of 0.5 mV s−1 in acetonitrile-based solution containing Another promising family of materials for MIBs alternative anodes is that of tin-based electrodes. Tin theoretically implies several advantages over bismuth as anode mate- rial [110]: (1) lower insertion potential; (2) higher theoretical specific capacity; (3) consider- ably lower atomic weight (118.71 u for tin vs. 208.98 u for bismuth); (4) Earth abundancy of tin is approximatively two orders of magnitude greater than that of bismuth. Furthermore, in the case of bismuth-based anodes each bismuth atom is able to exchange three electrons (see Equation (1)). As for tin-based anode, the anodic alloy is Mg2Sn and its chemical reaction allows an exchange of four electrons for each tin atom, one more than what occurs for each bismuth atom: Sn+2Mg2+ +4e− →Mg2Sn (2)PDF Image | Overview on Anodes for Magnesium Batteries
PDF Search Title:
Overview on Anodes for Magnesium BatteriesOriginal File Name Searched:
nanomaterials-11-00810.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |