
PDF Publication Title:
Text from PDF Page: 004
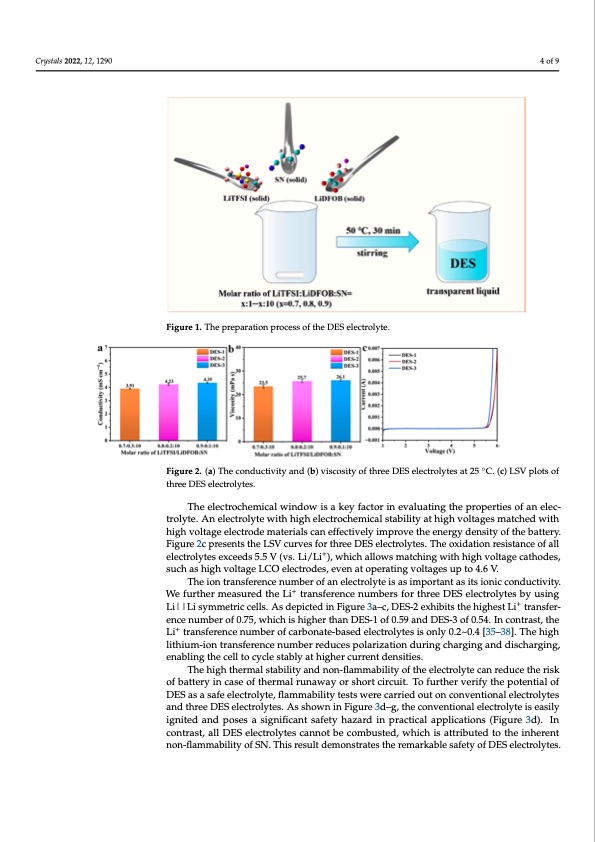
Crystals 2022, 12, 1290 4 of 9 Crystals 2022, 12, x FOR PEER REVIEW 4 of 10 Figure 1. The preparation process of the DES electrolyte. The electrochemical window is a key factor in evaluating the properties of an elec- trolyte. An electrolyte with high electrochemical stability at high voltages matched with high voltage electrode materials can effectively improve the energy density of the battery. Figure 2c presents the LSV curves for three DES electrolytes. The oxidation resistance of all electrolytes exceeds 5.5 V (vs. Li/Li+), which allows matching with high voltage cath- oFdigeus,resu1.cThhaesphriegpharvaotilotnagperoLcCesOs oefltehcetrDoEdSese,lecvtreonlyatte.operating voltages up to 4.6 V. Figure 1. The preparation process of the DES electrolyte. The electrochemical window is a key factor in evaluating the properties of an elec- trolyte. An electrolyte with high electrochemical stability at high voltages matched with high voltage electrode materials can effectively improve the energy density of the battery. Figure 2c presents the LSV curves for three DES electrolytes. The oxidation resistance of all electrolytes exceeds 5.5 V (vs. Li/Li+), which allows matching with high voltage cath- odes, such as high voltage LCO electrodes, even at operating voltages up to 4.6 V. ◦ Figure 2. (a) The conductivity and (b) viscosity of three DES electrolytes at 25 °C. (c) LSV plots of Figure 2. (a) The conductivity and (b) viscosity of three DES electrolytes at 25 C. (c) LSV plots of + Wtroelyfuter.thAenr emlecatsruolryetde twhiethLihigtrhaneslefecrtreoncheenmuimcabl esrtsabfoilritytharteehiDghESvoelteacgtreoslmytaetschbyeduwsiintgh + Lhi|g|hLvioslytamgme eltericctrcoedlles.mAastedrieaplsiccteadn einffeFcitgiuvreely3iam–cp,rDovEeSt-h2eexenheibrgitys dtheenshiitgyhoefsthLeibtartatnersy-. fFeirgeunrcee2ncupmrebserntosft0h.e75L,SwVhciuchrviesshfiogrhtehrrteheaDnEDSEeSle-1ctorofl0y.t5e9s.aTnhdeDoExSid-3atoiofn0.r5e4s.isItnancocentorfaastl,l three DES electrolytes. three DES electrolytes. The ieolnecttrraoncshfermenicael nwuimndboerwofisaan ekleyctfraocltyotre isnaesviamlupaotritnagnthaes iptsroiopneircticeosnodfuacntiveilteyc.- + tehlectLriolytrtaenssefxecreendces 5n.u5mVb(evrs.oLfi/caLribo),nwathei-cbhasaelldowelsecmtraotlcyhtiensgiws oitnhlyhi0g.h2~v0o.l4ta[g3e5–c3a8th].oTdhees, + Figure 2. (a) The conductivity and (b) viscosity of three DES electrolytes at 25 °C. (c) LSV plots of hstuhigrcehelaiDtshEhiSuigemlhe-cvitoroonlltytartgaeesn.sLfCerOeneclecnturomdbese,rerveednucaetsopoelrartiznagtivoonltdaugreisngupchtoar4g.i6nVg.and discharg- ing,eTnhaebliionngttrhaenscfelrletnocceynculemsbtaebrloyfaatnheiglehcetrocluyrtreeinstadseinmspitoiersta.ntasitsionicconductivity. + We fuTrhtheeironmteransusfrerdenthcenLuimtbrearnosfearneneclectnruomlytbeeirsafsoirmthproeretaDntEaSseitlseciotrnoilcyctoesndbuycutisviintyg. Li||Li symmetric cells. As de+picted in Figure 3a–c, DES-2 exhibits the highest Li+ transfer- We further measured the Li transference numbers for three DES electrolytes by using encenumberof0.75,whichishigherthanDES-1of0.59andDES-3of0.54.Incontra+st,the Li||Li symmetric cells. As depicted in Figure 3a–c, DES-2 exhibits the highest Li trans- Li+ transference number of carbonate-based electrolytes is only 0.2~0.4 [35–38]. The high ference number of 0.75, which is higher than DES-1 of 0.59 and DES-3 of 0.54. In contrast, lithium+-iontransferencenumberreducespolarizationduringcharginganddischarging, the Li transference number of carbonate-based electrolytes is only 0.2~0.4 [35–38]. The enabling the cell to cycle stably at higher current densities. high lithium-ion transference number reduces polarization during charging and discharg- The high thermal stability and non-flammability of the electrolyte can reduce the risk ing, enabling the cell to cycle stably at higher current densities. of battery in case of thermal runaway or short circuit. To further verify the potential of DES as a safe electrolyte, flammability tests were carried out on conventional electrolytes and three DES electrolytes. As shown in Figure 3d–g, the conventional electrolyte is easily ignited and poses a significant safety hazard in practical applications (Figure 3d). In contrast, all DES electrolytes cannot be combusted, which is attributed to the inherent non-flammability of SN. This result demonstrates the remarkable safety of DES electrolytes.PDF Image | Non-Flammable Dual-Salt Deep Eutectic Electrolyte
PDF Search Title:
Non-Flammable Dual-Salt Deep Eutectic ElectrolyteOriginal File Name Searched:
crystals-12-01290-v2.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |