
PDF Publication Title:
Text from PDF Page: 009
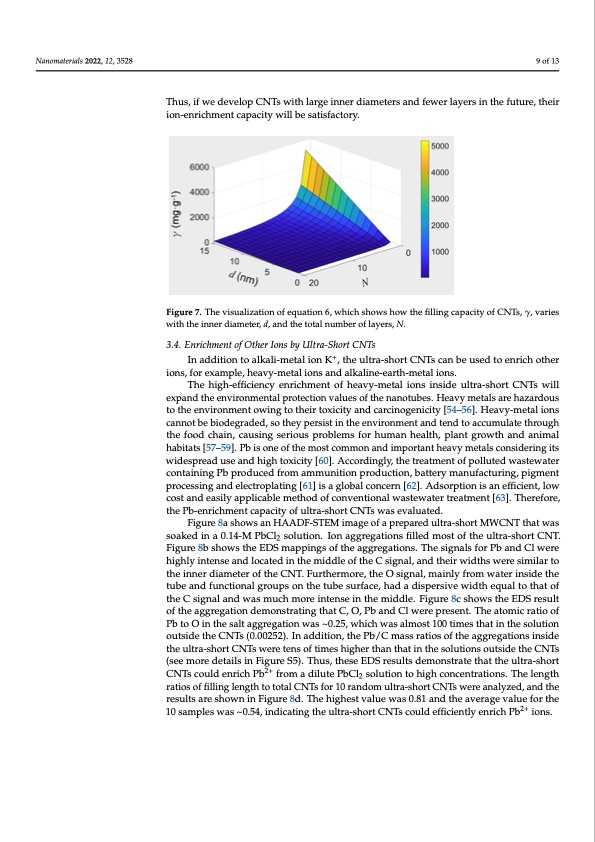
Nanomaterials 2022, 12, 3528 using equation 6. Figure 7 visualizes equation 6 when we assume the ultra-short CNTs have a space occupancy of 0.2. The ion-enrichment capacity increases with the inner diameter of CNTs, while it decreases with the increase in the total number of layers. For example, for MWCNTs with an average inner diameter of 7 nm and an average layer number of 20, and assuming the filling material is crystalline KCl with a relative density 9 of 13 of 1.98 and a space occupancy of 0.2 (this is just an assumption), the K-enrichment ca- pacity of CNTs is 30.9 mg/g. If the average value of the layer number of MWCNTs, N, can be decreased to 3, the K-enrichment capacity of the CNTs increases to ~354 mg/g. Thus, if we develop CNTs with large inner diameters and fewer layers in the future, their Thus, if we develop CNTs with large inner diameters and fewer layers in the future, ion-enrichment capacity will be satisfactory. their ion-enrichment capacity will be satisfactory. Figure 7. The visualization of equation 6, which shows how the filling capacity of CNTs, γ, varies Figure 7. The visualization of equation 6, which shows how the filling capacity of CNTs, γ, varies with the inner diameter, d, and the total number of layers, N. with the inner diameter, d, and the total number of layers, N. 33.4.4. .EnrriicchhmeennttooffOtthheerrIIoonnssbbyyUltltrraa--SShhoorrttCCNTTs s ++ IInnaadddititioionntotoaalklkaalil-im-meettaallioionnKK ,, tthe ullttra--short CNTs can beeusseedttooeennrricichhootthheerr ioionnss, ,ffoorreexxaampplele, ,hheeaavvyy-m-meetatal lioionnssaannddaalklkaalilninee-e-eaartrhth-m-meetatal lioionns.s. TThhee hhigighh--eefffifcicieiennccy eennrriicchmeentt ooff hheeaavvy--meettall iionss iinssiidee ullttrraa--sshorrtt CNTss wilill eexxppaannddthtehenevnivroirnomnemnetanltaplroptreocteiocntiovnaluveasluoefsthoef nthaneontuabneost.uHbesa.vHy emaevtyalsmaerteahlsazaarredhoauzs- taortdhoeuesnvtioronthmeenetnovwirionngmtoenthteiorwtoinxgicittyoanthdecirarctionxoigcietnyiciatnyd[54c–a5r6ci]n.oHgeanviyci-tmyet[a5l4i–o5n6s]. cHanenaovtyb-me betioaldieognrsadceadn,nsoot tbheeybipoedresgisrtaidnetdh,esoentvhieryonpmeresnisttaindthtenedntvoiraocncummenutlatnedthtreonudghto tahcecufomoudlactheaitnh,rocuaughsinthgeseforoioduschparionb,lceamusinfogrsheurimouasnphreoabltlhem,pslafonrthguromwatnhhaenadltha,nipmlaanlt habitats [57–59]. Pb is one of the most common and important heavy metals considering its growth and animal habitats [57–59]. Pb is one of the most common and important heavy widespread use and high toxicity [60]. Accordingly, the treatment of polluted wastewater metals considering its widespread use and high toxicity [60]. Accordingly, the treatment containing Pb produced from ammunition production, battery manufacturing, pigment of polluted wastewater containing Pb produced from ammunition production, battery processing and electroplating [61] is a global concern [62]. Adsorption is an efficient, low manufacturing, pigment processing and electroplating [61] is a global concern [62]. Ad- cost and easily applicable method of conventional wastewater treatment [63]. Therefore, sorption is an efficient, low cost and easily applicable method of conventional the Pb-enrichment capacity of ultra-short CNTs was evaluated. wastewater treatment [63]. Therefore, the Pb-enrichment capacity of ultra-short CNTs Figure 8a shows an HAADF-STEM image of a prepared ultra-short MWCNT that was was evaluated. soaked in a 0.14-M PbCl solution. Ion aggregations filled most of the ultra-short CNT. Figure 8a shows an HAADF-STEM image of a prepared ultra-short MWCNT that 2 Figure 8b shows the EDS mappings of the aggregations. The signals for Pb and Cl were was soaked in a 0.14-M PbCl2 solution. Ion aggregations filled most of the ultra-short highly intense and located in the middle of the C signal, and their widths were similar to CNT. Figure 8b shows the EDS mappings of the aggregations. The signals for Pb and Cl the inner diameter of the CNT. Furthermore, the O signal, mainly from water inside the tube and functional groups on the tube surface, had a dispersive width equal to that of the C signal and was much more intense in the middle. Figure 8c shows the EDS result of the aggregation demonstrating that C, O, Pb and Cl were present. The atomic ratio of Pb to O in the salt aggregation was ~0.25, which was almost 100 times that in the solution outside the CNTs (0.00252). In addition, the Pb/C mass ratios of the aggregations inside the ultra-short CNTs were tens of times higher than that in the solutions outside the CNTs (see more details in Figure S5). Thus, these EDS results demonstrate that the ultra-short CNTs could enrich Pb2+ from a dilute PbCl2 solution to high concentrations. The length ratios of filling length to total CNTs for 10 random ultra-short CNTs were analyzed, and the results are shown in Figure 8d. The highest value was 0.81 and the average value for the 10 samples was ~0.54, indicating the ultra-short CNTs could efficiently enrich Pb2+ ions.PDF Image | Ion Enrichment inside Ultra-Short Carbon Nanotubes
PDF Search Title:
Ion Enrichment inside Ultra-Short Carbon NanotubesOriginal File Name Searched:
nanomaterials-12-03528.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |