
PDF Publication Title:
Text from PDF Page: 008
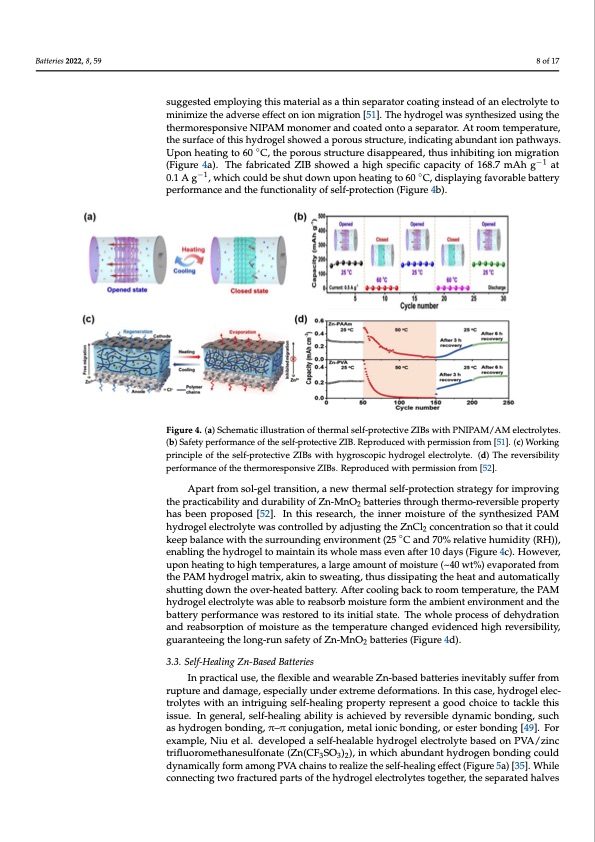
isopropylacrylamide (NIPAM) monomer for supercapacitors [50]. By controlling the ion Batteries 2022, 8, 59 migration inside the gel matrix through sol-gel transition, this thermoresponsive hydrogel electrolyte could deteriorate the electrochemical performance at high temperature and re- store it to the initial state after cooling to room temperature, manifesting a self-protection effect. However, the limited solubility of zinc salt in this thermoresponsive hydrogel elec- 8 of 17 trolyte adversely affected the specific capacity of the assembled battery. To improve the ionic conductivity of these thermoresponsive hydrogel materials, Zhu et al. suggested em- ploying this material as a thin separator coating instead of an electrolyte to minimize the suggested employing this material as a thin separator coating instead of an electrolyte to adverse effect on ion migration [51]. The hydrogel was synthesized using the ther- minimize the adverse effect on ion migration [51]. The hydrogel was synthesized using the moresponsive NIPAM monomer and coated onto a separator. At room temperature, the thermoresponsive NIPAM monomer and coated onto a separator. At room temperature, surface of this hydrogel showed a porous structure, indicating abundant ion pathways. the surface of this hydrogel showed a porous structure, indicating abundant ion pathways. Upon heating to 60 °◦C, the porous structure disappeared, thus inhibiting ion migration Upon heating to 60 C, the porous structure disappeared, thus inhibiting ion migration (Figure 4a). The fabricated ZIB showed a high specific capacity of 168.7 mAh g−1 a−t10.1 A (Figure 4a). The fabricated ZIB showed a high specific capacity of 168.7 mAh g at −1 g , which could be shut down upon heating to 60 °C, displaying favorable battery perfor- −1 ◦ 0.1 A g , which could be shut down upon heating to 60 C, displaying favorable battery mance and the functionality of self-protection (Figure 4b). performance and the functionality of self-protection (Figure 4b). Figure 4. (a) Schematic illustration of thermal self-protective ZIBs with PNIPAM/AM electrolytes. Figure 4. (a) Schematic illustration of thermal self-protective ZIBs with PNIPAM/AM electrolytes. (b) Safety performance of the self-protective ZIB. Reproduced with permission from [51]. (c) Work- (b) Safety performance of the self-protective ZIB. Reproduced with permission from [51]. (c) Working ing principle of the self-protective ZIBs with hygroscopic hydrogel electrolyte. (d) The reversibility principle of the self-protective ZIBs with hygroscopic hydrogel electrolyte. (d) The reversibility performance of the thermoresponsive ZIBs. Reproduced with permission from [52]. performance of the thermoresponsive ZIBs. Reproduced with permission from [52]. Apart from sol-gel transition, a new thermal self-protection strategy for improving Apart from sol-gel transition, a new thermal self-protection strategy for improving thteheprparcatcitciacabbiliiltiytyaanddurability of Zn-MnO2 batterriieesstthrroouugghhththeremrmo-or-erveevresirbslieblperpoproeprteyrty 2 hahsasbebenenprporpoopsoesded[5[25]2.].InInthitshirsesresaeracrhc,ht,htehienninenremr moiostiustruereofotfhtehseysnytnhtehseizsiezdedPAPAMMhy- hydrogelelectrolytewascontrolledbyadjustingtheZnCl concentrationsothatitcould drogel electrolyte was controlled by adjusting the ZnCl2 concentration so that it could keep balance with the surrounding environment (25 ◦C and 70% relative humidity (RH)), keep balance with the surrounding environment (25 °C and 70% relative humidity (RH)), enabling the hydrogel to maintain its whole mass even after 10 days (Figure 4c). However, upon heating to high temperatures, a large amount of moisture (~40 wt%) evaporated from the PAM hydrogel matrix, akin to sweating, thus dissipating the heat and automatically shutting down the over-heated battery. After cooling back to room temperature, the PAM hydrogel electrolyte was able to reabsorb moisture form the ambient environment and the battery performance was restored to its initial state. The whole process of dehydration and reabsorption of moisture as the temperature changed evidenced high reversibility, guaranteeing the long-run safety of Zn-MnO2 batteries (Figure 4d). 3.3. Self-Healing Zn-Based Batteries In practical use, the flexible and wearable Zn-based batteries inevitably suffer from rupture and damage, especially under extreme deformations. In this case, hydrogel elec- trolytes with an intriguing self-healing property represent a good choice to tackle this issue. In general, self-healing ability is achieved by reversible dynamic bonding, such as hydrogen bonding, π–π conjugation, metal ionic bonding, or ester bonding [49]. For example, Niu et al. developed a self-healable hydrogel electrolyte based on PVA/zinc trifluoromethanesulfonate (Zn(CF3SO3)2), in which abundant hydrogen bonding could dynamically form among PVA chains to realize the self-healing effect (Figure 5a) [35]. While connecting two fractured parts of the hydrogel electrolytes together, the separated halvesPDF Image | Flexible Zn-Based Batteries with Polymer Electrolyte
PDF Search Title:
Flexible Zn-Based Batteries with Polymer ElectrolyteOriginal File Name Searched:
batteries-08-00059.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |