
PDF Publication Title:
Text from PDF Page: 005
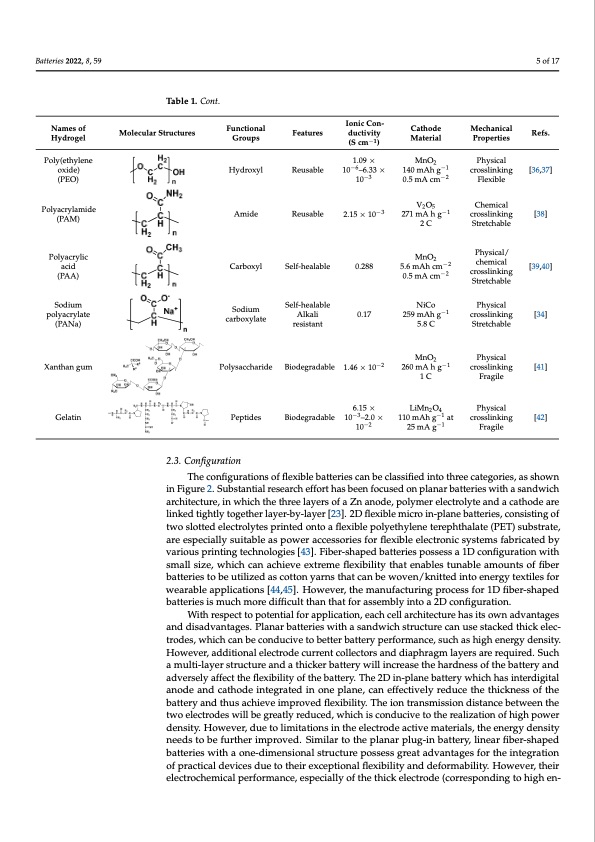
Names of Hy- Ionic C−1 on- Functional cm ) Cathode Mechanical Properties drogel Names of Hy- Table 1. Some representatGiverpooulypmser electrolytes for Zn-baseIdonbaitcterCieos.n- Material Polyvinyl al- Features ductivity (S Functional Ionic Con- Cathode Cathode Mechanical Names of Hy- drogel Molecular Structures Molecular Structures Molecular Structures Molecular Structures Table 1. Cont. Groups Ionic Con-Ionic Con- Menchaannoicraolds crosslink- Material Properties coNhaomles of Hy- Names ofdrHogye-l Functional Features ductivity (S Cath−1ode Mechanical Refs. PProCplaeyrtathineosidlinee MPehcyhsaincaiclal Polyvinydlroagl-el d(PrVogAe)l Groups Groups Featurcems ) cm−1) nManaoterroidals Pcrospselirntike-s −1 Batteries 2022, 8, 59 cohol −1 cm ) Po0.l1yaAnigline StPrhetycshiacable Physical −1 ing [35] PolyviPnoylylvainl-yl al- 0.P0o5ly–a1n2il.i6ne 1P2h3ysmicaAl h g crnosasnlinokr-ods crosslink- Polyvinyl al- (PVA) Polyaniline nanorods Physical 0 i . n M1 g n A O g 2 [ 3 − 5 1 ] S t r e t i c n h g a b l e cohol cohol [35] [3[63,53]7] Polly(vetinhycloeahnlo-el (PVA) Hydroxyl Self-healable 0.05–12.6 123 mAh g−1 123ingmAh g nanorods crosslink- crosslink- (PVA) ocxoihdoe(l)PVA) Hydroxyl SeRlfe-uhseablaleble −6 −1 123 mAh g Stretchable S1tr4e0tc.1hmaAbAlegh g Poly(ethylene 6.33 × 10 −3 123MmnAOh2 g−1 Physical −2 (PVEOA) P o l y ( e t h y l e n e 2 1 . 0 9 M× n1 O0 0.5 mA cm Physical crosslink- oxide) Poly(ethylene Ionic Con- −6 Cathode PMFhleycxhsiaibcnlaieclal cro0s.s1linAk-g−1 −1 Stretchable Names of Functional −6 Reu1s.a0b9l×e10 – MnO2 Poly(ethyolxeinde) Features ductivity Groups 1.09×10 –6.13430m×A10hg Hydrogel Material Properties oxide) (PEO) 6.33 × 10−3(S cm−1) −6 −1 crosslink- [36−,237] oxide()PEO) PPoolyly(eatchry(PleaEn-Oe) 6.33 × 10 Reusable 140 mAh g 0.5 mA cm CPheymsiicall ing [36,37] Poly(ethylene 1.09 × Physical (PEO) omxidodxei)de) 1.09 × 10−6– 2 1C40hmemAihcagl −1 crosslink- Polyacryla- −3V2O5 −3 61.033 × 10 Chemical Chemical ing ing −1 Polyac(PrEyOla)- Polyacryla- V2O5 271 mA h g −2 0c.5romssAVlin2cOkm-5 Flexible ((PAEMO)mide midemide −2 0.5 m2ACcm Polyacry(PlAa-M) Amide Reusable −3−1 22.7115m×A1h0 g ing [38] (PAM) P(PolAyaMcry)lamide 2 C Stretchable− 1 crosslink- mide Amide Reusable 2.15 × 10 MnO2 −3 CPcrhoyessmslincikacialn/lg [38] Stretchable [38] (PAM) 271 mA h g PhyMsVicn2aOlO/ 52 ing Polyacryla- 2 C Stretchable Polyacrylic (PAM) Physical/ 2 C crosslink- chemical midPoelyacrylic Amide Reusable MnO2 −3 2.15 × 10 Stretchable [38] Polyacrylic acid acid Carboxyl Self-healable Carboxyl Self-healable 0.288 Carboxyl Self-healable 0.288 ing chemical crosslink- [39,40] (PAM)acid Polyacrylic cm 0.5 mA −2 −2 cm 20.C5mA Polya(cPrAylAic) (PAA) cm 0.5mA cm−2 chemical MinngO SPtrheyitncshgicabl/le (PAA) acidacid Carboxyl Self-healable 0.288 −2 McmnO2 Polyacrylic cm Stretchable (PAA) a c i d S e l f - h e a l a b l e 0 . 2 8 8 NiCo −1 − 1 5.6 mAh P h y Ms i c n a Ol − 2 2 c r o s s l i n k - (PAA) S0c.t5rmemtcAha0cb.m5lemA Sctrheetmchiacbale Sodium poly- Polyacrylic cm Phy−s2ical Physical Sodium poly- Sodium poly- Sodium car- Self-healable Self-healable NiCo 259 mAh g Stretchable (PAAa)crylate 0.17 Sodium car- AlkaSlierlef-healable NiCo −1 cro5ss.6linmk-Ah[34] ing Sodium NiCo Physical acid acrylate boSxSoyColdaitureubmoxcyalr- Self-healable 0.288 259 mAh g 0.17 ing −2 crosslink- acproylylactreylate (PANa) Alkali 05.1.87C 5.8 C −1 [34] 259 imn−2gAh g −1 cm 0.5 mA [34] (PA(PA(PN)AaN) a) Sodium poly- carboxylate Self-healable sistant Alkali re- Self-healable NiCo Stretchabl−e2 5c.m8 C (PANa) 5.8 C Stretchable acrylate Sodium poly- Sodium car- boxylate 0.17 MnO2 259 mAh g−1 Physical crosslink- StPrhetycshiacaable [34] (PANa) acrylate MnO2 crosslink- 5.8 C PhPhyyssiical Stretchable P h i y n s g i c a l Xanthan gum Sodium poly- Alka1l.i46re×-10 −2 0.17 260 mA h g MnO2 −1 c 2 r 5 o s 9 s l mi n k A - h g [ 4 1 ] [ 3 4 ] crosslinking [41] XaXntahnatnhagnugmum (PANa) − 2 − 2 Polysbaoccxhyarliadte Biodegra1d.4a6bl×e101.46 × 10 acrylate Xanthan gum (PANa) Polysaccha- Biodegrada- Fragile −1 crosslink- Stretchable [34] Xanthan gum 2O0 260 mA h g [41] Gelatin Xanthan gum Xanthan gum Gelatin Gelatin Gelatin Gelatin ble 2.0 × 10−2 PepPtoidleyssaccha- Biodegrada- −2 −1 110 mAh g 10 −2−1 −1 260 mA h g Fragile Molecular TSatbrlue 1c.tSuormeesrepresentative polymer electrFoleyatetsuforreZsn-badseud cbtaittveiriteys. (S Table 1. Some representative polymer electrolytes for Zn-based batteries. Refs. Refs. [35] Refs. 5 of 17 Molecular Structures Hydroxyl [36,37] 140 mAh g Refs. 2.3. Configuration 110 mAh g −1 ing [42] Physical 2.3. Configuration i n g Functional cm−1) Polyaniline Physical Hydroxyl Self-healable 0.05–12.6 GrFoupnsctional cMmate)rial Hydroxyl Reusable 1.09×10 – Hydroxyl HydAromxyidl e Hydroxyl −3 −2 −1 0.5mAc−m1 −2 Flexible Amide Reusable 2.15 × 10 crosslink- ing [3−81]SCtFrheletcxmhibiaclbeale −3 Amide Reusable 2.15 × 10−3 −1 crosslink- [38] 271 mA h g inVg 2O5 Amide Reusable 271 mA h g Carboxyl Self-healable 0.288 5.6 mAh cm [39,40] C a r b o x y l Self-healable [ 3 9 , 4 0 ] crosslink- [39,40] boxylate sistant crosslinking [34] Polysaccha- Biodegrada- i n Ng i C o − 1 −1 260 mA h g [41] RReuesuasbaleble Reusable −6 10 2.–165.3×3 ×10−3 271 mA h g 140 mAh g crosslinking [3[836],37] Flexible [36,37] Alkali re- 0.17 cm Alkali re- boxylatesistantresistant 259 mAh g Stretchable StPrhetycshicaablle ing Sodium car- Polysaccha- Biodegradas-istant NiCo Physical ing crosslink- crosslink- ride bleSelf-healable rSidoedium carb-le sistant 260 mA h g 1 C 1 C 0.17 inMg nO2 5.8 C boxylate Alkali re- 1.46 × 10−2 259 mAh g Fragile ing [41] Physical ride ble sistant 260 mA h g −1 ing Sctroestcshlianbkl-e Polysaccha- Biodegrada- LiMn2O4 6.15 × −2 Physical 1 C PFhPrhyaysgsiclceal GelGaetilnatin PepPteipdterisdideseBiodegbraldeable−310–2.0×−11c0romsAsMlihngkO-2at[42]crosinslginking[42] 2.3. Configuration ble 2.0 × 10 Biodegrada- 6.15 × 10−3– 1.4L6iM×n1 Biodegrada- 6.15 × 10 – 110 mAh g ing 25 imngA g crosslink- ble 2.0 × 10 ble 1.46 × 10 at 25 mA g −2 Polysaccha- Biodegrada- −1 260 mA h g−1 Phiynsgical ride Perpitdidees ble −1 Fragile MnO2 Biodegrada- −−32 61.1.456××1100 – −2 1 C crosslink- Fragile [41] L i M1 nC2 O 4 The configurations of flexible batteries can be classified into three categorie−s1, as Refs. ing ducMtiavtietryial(S P1r2op3emrtiAesh g Features ductivity (S −1 −1 Hydroxyl Hydroxyl Self-healable 0.05–12.6 nanorods cProosslylinakn-ili−n1[3e5] Physical Self-healable Hydroxyl Self-healable 0.05–12.6 1.09 × 10 0.1 A g – −1 0.05–12. 0.1 A g −1 −1 StPrhetycshicaablle – Hydroxyl Reusable −6 −3−1 crossMlinkO- 2[36,37] ing −2 60.3.53m×A1c0m−3 MV2nO52 FlMexnibOle 2 C 2 C − 3 2.15 × 10 5.6 mAh 0.288 StreVtc2hOa5ble Chemical 5.6 mAh −2 crosMslinkO- 2[39,40] at 25 mA g Physical Fragile [41] −3 at 25 mA g crForsasglinlek- TheTcohneficgounrafitigonusraotfioflnexsibolfeflbeBaxtitoiebrdilesgbcrantdtebare-iecsl6a.cs1sa5infi×ebd1e0incltoa–stshirfieedcaitnegtortihesr,eaescategories,asshown shown in Figure 2. Substantial research effort has been focused on planar batteries w−i1th aPFhryasgicleal Peptides 110 mAh g [42] −2 LiMn2O4 ing shown in Figure 2. Substantial research effort has been focused on planar batteries with a in Figure 2. Substantial research ebffloert has be2e.0n ×fo1c0used on planar batteries with a sandwich sandwich architecture, in which the three layers of a Zn anode, polymer electrolyte and a crosslink- sandwich architecture, in which the three layers of a Zn anode, polymear tel2ec5trmolyAtegand a Biodegrada- 6.15 × 10−3– −1 catahrocdheiatreecltiunkred, itnighwtPlhyeiptcothgiedtheeser tlahyrere-blya-lyaeyresr [o23f]a. 2ZDnfleaxniboledme,icproliyn-mpleanreebleatctte−r1oielsy,Ptehyasnidcaal cath[4o2d]e are 110 mAh g Fragile −2 ing 2ca.3th.oCdoenarfiegluinrkaetdiotinghtly together layer-by-blalyeer [23]. 2D2f.l0ex×ibl1e0micro in-LpilManenb2Oatt4eries, consisting of two slotted electrolytes printed onto a flexible polyethylene terephthalate linked tightly together layer-by-layer [23]. 2D flexib−l3e micro in-plan−e1 bcartotesrsileins,kc-onsisting of Biodegrada- 6.15 × 10 – at 25 mA g consisting of two slotted electrolytes printed onto a flexible polyethylene terephthalate (PET) substrate, are espPeecipaltliydseusitable as power accessories for flexible electronic syst−e1ms [42] The configurations of flexible batteries can be clas1s1i0fiemdAihntgo thrFeeracgailtegories, as twoslottedelectrolytesprintedontoaflexiblepoly−e2thyleneterephthalatieng(PET)substrate, (PET) substrate, are especially suitable as pobwler accessori2e.s0fo×r 1fl0exible electronic systems 2fa.3b.riCcaotendfibgyuvrartiouns printing technologies [43]. Fiber-shaped batteries possess a 1D con- −1 shown in Figure 2. Substantial research effort has been foacut 2se5dmoAn pglanar batteries with a fabarirceatedspbyecviaarliolyusspurintatibngleteacshnpoolowgiesr[a43c]c.eFsibseorr-siheaspfeodrbafltetexriebslpeoesslescstrao1nDicosny-stemsfabricatedby figuration with small size, which can achieve extreme flexibility that enables tunable Fragile s f i a g n u r d a T wt i o h i n c e h w c a i o t r h n c f h s i m i g t a u e l c l r t a s u t i z i r o e e , n , w s i n h o i wc f h h f c l i a e c n h x i a b t c h l h e e i e v t b h e a r t e e t x e e t r r l e i a me y s e e c r f l s a e x n o i b f b i a l i e t Z y c n t l h a a a s t n s e o i f n d i a e e b d , l e p s i o n t u l t y o n ma b t h e l e r r e e e l e c c a t r t o e l g y o t r e i e a s n , d a a s 2a.m3vo.auCrnoitonsufoifsgfupibreairtnibtoianttgeritescthonboelougtiliiezsed[4a3s]c.oFttiobneyr-asrnhsatpheadt cbanatbterwieosvepno/ksnsiettsesdain1toDconfigurationwith amounts of fiber batteries to be utilized as cotton yarns that can be woven/knitted into shown in Figure 2. Substantial research effort has been focused on planar batteries with a cathode are linked tightly together layer-by-layer [23]. 2D flexible micro in-plane batteries, small size, which can achieve extreme flexibility that enables tunable amounts of fiber The configurations of flexible batteries can be classified into three categories, as s2a.3n.dCwonicfhigaurcahtiiotnecture, in which the three layers of a Zn anode, polymer electrolyte and a conbsaitstteirnigesotfotbweoustilloitzted aesleccottrtolnytyeasrpnrsinthteadt coantobeawfloevxeibnl/ekpnoitltyeedthiynlteonenteerrgeyphtetxhtaileastefor shown in Figure 2. Substantial research effort has been focused on planar batteries with a cathode are linked tightly together layer-by-layer [23]. 2D flexible micro in-plane batteries, (PEwTeT)ashruaebcsletornaftpiegp, ulaircaeatteiiosonpnsesco[ia4fl4lf,y4le5sx]ui.bitHlaebolwbeaeatvtseprio,ewtshecramancacbneussfcoalcraitseussrififinoegrdfpliernxotiocbelstehserfleoeecr t1croaDtnefiigcboseryrise-stseh, mapsed sandwich architecture, in which the three layers of a Zn anode, polymer electrolyte and a consisting of two slotted electrolytes printed onto a flexible polyethylene terephthalate fsahbobrwaictntaetirenideFsbiigysuvmraeur2ico.huSmsubposrtienadntiitnfifiaglcturelctshetnhaoraclnohgtehifeafsto[fr4ot3rh]a.sFsibebemerbn-slfyhoaicnputesodeadb2oaDtntepcroliaennsfiapgruobsrasaettiseosrnia.e1sDwictohna- cathode are linked tightly together layer-by-layer [23]. 2D flexible micro in-plane batteries, (PET) substrate, are especially suitable as power accessories for flexible electronic systems fsiagnudrwatiWcohnitahwrcrihethsitpesecmctutatrloel,psiionztewe,nhtwiachlifctohreactpahnprleiaeccalhtaiyoeenvr,eseeoaxcfhtarecZmenlleanfrlcoehdxiietbe,icpltiuotylryemthaeasrt eietlsnecaotbwrloenlsyatdteuvananandbtaleages consisting of two slotted electrolytes printed onto a flexible polyethylene terephthalate fabricated by various printing technologies [43]. Fiber-shaped batteries possess a 1D con- acamtahonoudnedtsiasoraefdlfviinbakneertadbgtaeitgst.herPtlilyeasntotaogrebtheateutretlriaileiyzserwd-biatyhs-lacaoystaetrnon[d2w3y]ia.cr2hnDstftrlhueaxctitbuclraenmcbaicenrowusionev-septnala/cknkneidbttatethdtiecriknietesol,ec- (PET) substrate, are especially suitable as power accessories for flexible electronic systems figturroadteiosn, wwhitch csamnabllesciozne,duwchivicehtocabnettaecrhbieavttereyxptreermfoermflaenxcibe,ilsituychthaastheignhabelneesrgtuyndaebnlesity. consisting of two slotted electrolytes printed onto a flexible polyethylene terephthalate fabricated by various printing technologies [43]. Fiber-shaped batteries possess a 1D con- amHouowntesvoefr,faibdedritbioanttaelrieelesctorobdeucutirlirzeendt caosllceocttoorns aynadrndsiathpahtracgamn blaeywerosvaerne/rkenqiuttierdedi.nStouch (PET) substrate, are especially suitable as power accessories for flexible electronic systems figuration with small size, which can achieve extreme flexibility that enables tunable a multi-layer structure and a thicker battery will increase the hardness of the battery and fabricated by various printing technologies [43]. Fiber-shaped batteries possess a 1D con- amounts of fiber batteries to be utilized as cotton yarns that can be woven/knitted into adversely affect the flexibility of the battery. The 2D in-plane battery which has interdigital figuration with small size, which can achieve extreme flexibility that enables tunable anode and cathode integrated in one plane, can effectively reduce the thickness of the amounts of fiber batteries to be utilized as cotton yarns that can be woven/knitted into battery and thus achieve improved flexibility. The ion transmission distance between the two electrodes will be greatly reduced, which is conducive to the realization of high power density. However, due to limitations in the electrode active materials, the energy density needs to be further improved. Similar to the planar plug-in battery, linear fiber-shaped batteries with a one-dimensional structure possess great advantages for the integration of practical devices due to their exceptional flexibility and deformability. However, their electrochemical performance, especially of the thick electrode (corresponding to high en- − ing ing 1 6 4 −1 1 C [42] −3 −1 ing 0 . 5 i n mg A c m 1F4le0xmiblAe h g chemical −1 2751.6mmAAhhg Physical/ crosslink- [39,40] Physical/ 2 5in.6g m−A2−h2 chemical crosslink- [39,40] −2 − 2 crosslinking crmossli0nk.5- mA chemical 1 C Fragile M5.n8OC2 PLhiyMsnicaOl crosslin2 k-4 Fragile LiMn2O4 crosslink- Physical/ StrientcghablePDF Image | Flexible Zn-Based Batteries with Polymer Electrolyte
PDF Search Title:
Flexible Zn-Based Batteries with Polymer ElectrolyteOriginal File Name Searched:
batteries-08-00059.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |