
PDF Publication Title:
Text from PDF Page: 005
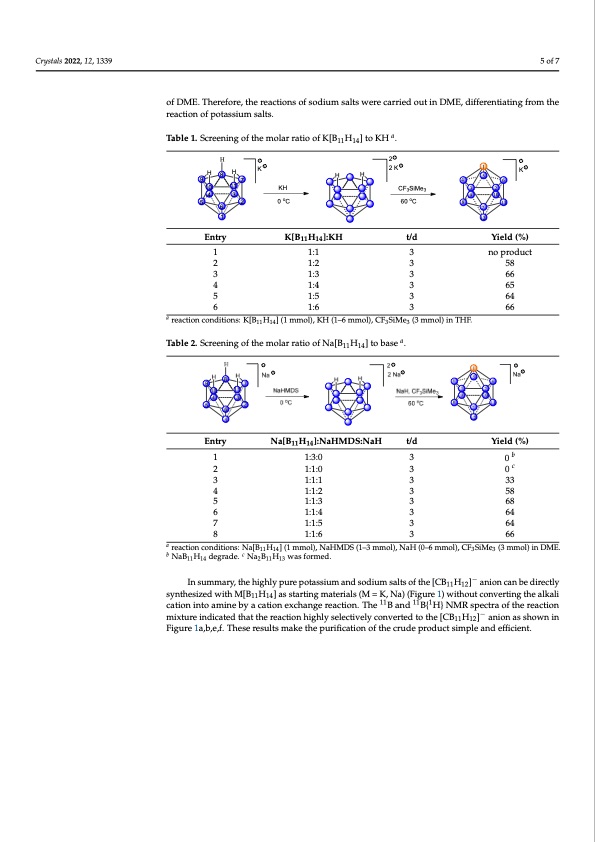
hydrogen from Na[B H ] to form Na [B H ], but NaHMDS would continually react Crystals 2022, 12, 1339 of Na[B11H14] to NaHMDS to NaH (Table 2). After a series of screenings, the 1:1:3:3 molar ratio of Na[B11H14] to NaHMDS to NaH to CF3SiMe3 was used in the overall preparation 5 of 7 11 14 2 11 13 with the formed Na2[B11H13], resulting in a decomposition to unidentified small boron with the formed Na2[B11H13], resulting in a decomposition to unidentified small boron cages. In considering that NaH can react with CF3SiMe3 to provide carbene:CF2, thus, we cages. In considering that NaH can react with CF3SiMe3 to provide carbene:CF2, thus, we selected the combination of NaHMDS and NaH, and screened the different molar ratios selected the combination of NaHMDS and NaH, and screened the different molar ratios of Na[B11H14] to NaHMDS to NaH (Table 2). After a series of screenings, the 1:1:3:3 molar ratio of Na[B11H14] to NaHMDS to NaH to CF3SiMe3 was used in the overall preparation of Na[CB11H12]. Furthermore, it is worth noting that when we examined DME as a solvent, of Na[CB11H12]. Furthermore, it is worth noting that when we examined DME as a solvent, the yields were relatively higher than those in THF, probably because the slight polarity the yields were relatively higher than those in THF, probably because the slight polarity of DME. Therefore, the reactions of sodium salts were carried out in DME, differentiating ofDME..TThhereerfeofroer,eth,tehrearcetaiocntisoonfssodfisuomdisuamltsswaletrsewcaerreiecdaoruriteidnoDuMtEin,dDifMferEe,ndtiiaftfienrgenfrtoimatitnhge from the reaction of potassium salts. reaction of potassium salts. from the reaction of potassium salts. Table 1. Screening of the molar ratio of K[B11H14] to KH a. a H2 HHHK 2K 1K 9H8H7K H H 2K 4 15 6K 9108117 KH HH CF3SiMe3 43526 Table 1.. Screening of the mollar rattiio off K[[B11H14]]ttoKHa. . 11 14 4 113 9 10 10KH CFSiMe32 o 3o3 54 32 0C 60C 89 1011 5 6 2 0oC 60oC 8 7 11 67 Entry EEnnttrry K[B11H14]:KH K[BKH[B11]H:K1H4]:KH t/d t/d t/d Yield (%) YYieiledld(%()%) 4 3 65 1 1:1 1:2 1:3 1:4 1:5 1:6 1:1 1:2 1:2 1:3 3 3 58 2 no product 22 3 3 66 3 3 66 3 4 4 1:3 366 5 1:5 1:5 364 5 3 64 3 3 6666 5 6 1:6 1 12 1 12 1 1 11 141:1 3 no product no product a 6 1:6 3 66 reaction conditions: K[B H ] (1 mmol), KH (1–6 mmol), CF SiMe (3 mmol) in THF. 3 3 3 5858 1:4 1:4 365 3 65 3 64 a reaction conditions: K1[1B114H14] (1 mmol), KH (1–6 mmo3l), CF33SiMe3 (3 mmol) in THF. a reaction conditions: K[B11H14] (1 mmol), KH (1–6 mmol), CF3SiMe3 (3 mmol) in THF. Table 2. Screening of the molar ratio of Na[B H ] to base a. Table 2. Screening of the molar ratio of Na[B111H14] to base a. Table 2. Screening of the molar ratio of Na[B11H14] to base a. Entry Na[B11H14]:NaHMDS:NaH t/d t/d Yield (%) Entry Na[B11H14]:NaHMDS:NaH t/d Yield (%) Entry Na[B11H14]:NaHMDS:NaH Yield (%) bc 1 1:3:0 b0 b 3 c0 c 1 1:3:0 2 1:1:0 2 1:1:0 3 2 1:1:0 3 1:1:11:1:1 3 3 1:1:1 4 1:1:2 3 58 4 1:1:2 3 58 45 1:1:31:1:2 3 3 6858 5 1:1:3 3 68 6 1:1:4 3 64 5 1:1:3 3 68 6 1:1:4 3 64 7 1:1:5 3 64 6 1:1:4 3 64 78 1:1:61:1:5 3 3 6664 7 1:1:5 3 64 3 3 0b 0 3 0c 3 3333 3 33 1 1:3:0 3 0 a reactioncon8ditions:Na[B11H14](1mmol),N1a:1H:M6DS(1–3mmol),NaH(0–6m3mol),CF3SiMe3 (3mm66ol)inDME. a NaB11H14 d8egrade. Na2B11H13 was forme1d:1. :6 3 66 reaction conditions: Na[B11H14] (1 mmol), NaHMDS (1–3 mmol), NaH (0–6 mmol), CF3SiMe3 (3 a reaction conditbions: Na[B11H14] (1 mc mol), NaHMDS (1–3 mmol), NaH (0–6 mmol), CF3SiMe3 (3 mmol) in DME. NaB11H14 degrade. Na2B11H13 was formed. mmol) in DME. b NaB11H14 degrade. c Na2B11H13 was formed. − In summary, the highly pure potassium and sodium salts of the [CB11H12] anion can be directly synthesizedwithM[B H ]asstartingmaterials(M=K,Na)(Figure1)withoutconvertin−gthealkali Insummary,th11eh14ighlypurepotassiumandsodiumsaltsofthe[CB11H12] anioncan 11111 − cationIninstuomaminaeryb,ythaecahtigonhleyxcphuarnegpeoretasctsiiounm. Tahned sBodaniudmBsa{lHts}oNfMthRes[CpeBc1t1rHa o12f]thaenrioeanctciaon be directly synthesized with M[B11H14] as starting materials (M = K, Na) (Figure 1) without − mbeixdtuirecitnldyiscaytnedthtehsaitztehde wreaitchtioMn[hBi1g1hHly14s]ealsecsttivaertlyincgonmvaertteerdiatlost(hMe [=CKB , NHa)](Faignuiornea1s)swhoitwhnouint 111211 111 converting the alkali cation into amine by a cation exchange reaction. The B and B{ H} Figure 1a,b,e,f. These results make the purification of the crude product simple and11efficient1.1 1 converting the alkali cation into amine by a cation exchange reaction. The B and B{ H}PDF Image | Efficient Way to Directly Synthesize Unsolvated Alkali Metal
PDF Search Title:
Efficient Way to Directly Synthesize Unsolvated Alkali MetalOriginal File Name Searched:
crystals-12-01339.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |