
PDF Publication Title:
Text from PDF Page: 098
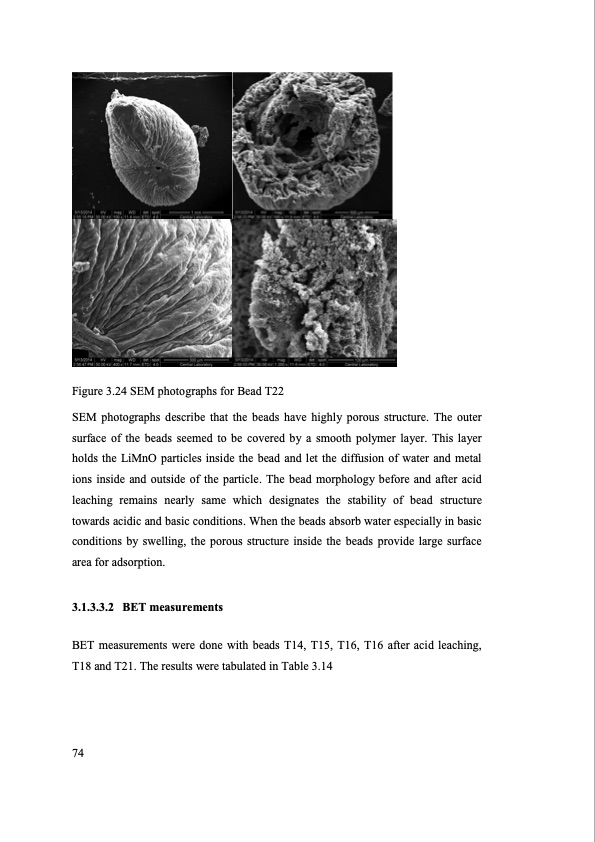
Figure 3.24 SEM photographs for Bead T22 SEM photographs describe that the beads have highly porous structure. The outer surface of the beads seemed to be covered by a smooth polymer layer. This layer holds the LiMnO particles inside the bead and let the diffusion of water and metal ions inside and outside of the particle. The bead morphology before and after acid leaching remains nearly same which designates the stability of bead structure towards acidic and basic conditions. When the beads absorb water especially in basic conditions by swelling, the porous structure inside the beads provide large surface area for adsorption. 3.1.3.3.2 BET measurements BET measurements were done with beads T14, T15, T16, T16 after acid leaching, T18 and T21. The results were tabulated in Table 3.14 74PDF Image | SEPARATION OF LITHIUM FROM BRINES
PDF Search Title:
SEPARATION OF LITHIUM FROM BRINESOriginal File Name Searched:
separation-lithium-from-brine.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |