
PDF Publication Title:
Text from PDF Page: 048
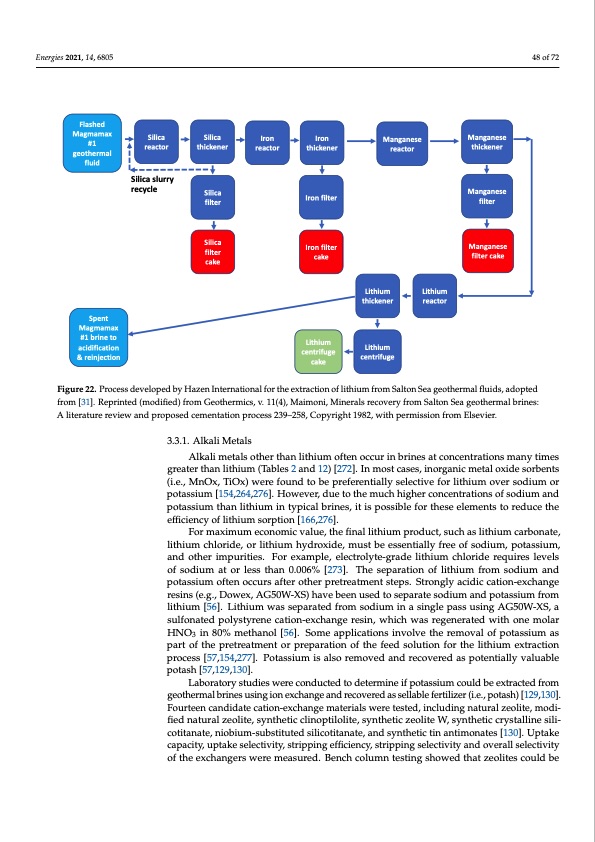
Energies 2021, 14, 6805 Typical applications of lithium sorbents for extraction of lithium from geothermal brine envision multiple steps, in addition to silica removal [76,77,78]. Figure 22 shows the complexity of the processes required to separate other minerals from lithium in treatment of geothermal brines [31,274,275]. Typical steps include removing alkaline earth metals from the brine; passing the treated brine through an ion-exchange column containi4n8goaf 72 molecular sieve lithium sorbent; eluting the lithium ions from the molecular sieve using a strong acid solution; and collecting the resulting lithium-rich eluate fluid from the ion- exchange reactor column [128]. Flashed Magmamax #1 geothermal fluid Silica reactor Silica slurry recycle Silica thickener Silica filter Silica filter cake Iron reactor Iron thickener Iron filter Iron filter cake Lithium centrifuge cake Manganese reactor Manganese thickener Manganese filter Manganese filter cake Lithium thickener Lithium centrifuge Lithium reactor Spent Magmamax #1 brine to acidification & reinjection Figure 22. Process developed by Hazen International for the extraction of lithium from Salton Sea geothermal fluids, adopted Figure 22. Process developed by Hazen International for the extraction of lithium from Salton Sea geothermal fluids, from [31]. Reprinted (modified) from Geothermics, v. 11(4), Maimoni, Minerals recovery from Salton Sea geothermal brines: adopted from [31]. Reprinted (modified) from Geothermics, v. 11(4), Maimoni, Minerals recovery from Salton Sea A literature review and proposed cementation process 239–258, Copyright 1982, with permission from Elsevier. geothermal brines: A literature review and proposed cementation process 239–258, Copyright 1982, with permission from Elsevier. 3.3.1. Alkali Metals 3.3.1. Alkali Metals Alkali metals other than lithium often occur in brines at concentrations many times Alkali metals other than lithium often occur in brines at concentrations many times greater than lithium (Tables 2 and 12) [272]. In most cases, inorganic metal oxide sorbents greater than lithium (Tables 2 and 12) [272]. In most cases, inorganic metal oxide sorbents (i.e., MnOx, TiOx) were found to be preferentially selective for lithium over sodium or (i.e., MnOx, TiOx) were found to be preferentially selective for lithium over sodium or potassium [154,264,276]. However, due to the much higher concentrations of sodium and potassium [154,264,276]. However, due to the much higher concentrations of sodium and potassium than lithium in typical brines, it is possible for these elements to reduce the potassium than lithium in typical brines, it is possible for these elements to reduce the efficiency of lithium sorption [166,276]. efficiency of lithium sorption [166,276]. For maximum economic value, the final lithium product, such as lithium carbonate, For maximum economic value, the final lithium product, such as lithium carbonate, lithium chloride, or lithium hydroxide, must be essentially free of sodium, potassium, lithium chloride, or lithium hydroxide, must be essentially free of sodium, potassium, and and other impurities. For example, electrolyte-grade lithium chloride requires levels other impurities. For example, electrolyte-grade lithium chloride requires levels of of sodium at or less than 0.006% [273]. The separation of lithium from sodium and sodium at or less than 0.006% [273]. The separation of lithium from sodium and potassium potassium often occurs after other pretreatment steps. Strongly acidic cation-exchange often occurs after other pretreatment steps. Strongly acidic cation-exchange resins (e.g., resins (e.g., Dowex, AG50W-XS) have been used to separate sodium and potassium from Dowex, AG50W-XS) have been used to separate sodium and potassium from lithium [56]. lithium [56]. Lithium was separated from sodium in a single pass using AG50W-XS, a Lithium was separated from sodium in a single pass using AG50W-XS, a sulfonated sulfonated polystyrene cation-exchange resin, which was regenerated with one molar polystyrene cation-exchange resin, which was regenerated with one molar HNO3 in 80% HNO3 in 80% methanol [56]. Some applications involve the removal of potassium as methanol [56]. Some applications involve the removal of potassium as part of the part of the pretreatment or preparation of the feed solution for the lithium extraction pretreatment or preparation of the feed solution for the lithium extraction process process [57,154,277]. Potassium is also removed and recovered as potentially valuable potash [57,129,130]. Laboratory studies were conducted to determine if potassium could be extracted from geothermal brines using ion exchange and recovered as sellable fertilizer (i.e., potash) [129,130]. Fourteen candidate cation-exchange materials were tested, including natural zeolite, modi- fied natural zeolite, synthetic clinoptilolite, synthetic zeolite W, synthetic crystalline sili- cotitanate, niobium-substituted silicotitanate, and synthetic tin antimonates [130]. Uptake capacity, uptake selectivity, stripping efficiency, stripping selectivity and overall selectivity of the exchangers were measured. Bench column testing showed that zeolites could bePDF Image | Recovery of Lithium from Geothermal Brines
PDF Search Title:
Recovery of Lithium from Geothermal BrinesOriginal File Name Searched:
energies-14-06805-v2.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |