
PDF Publication Title:
Text from PDF Page: 026
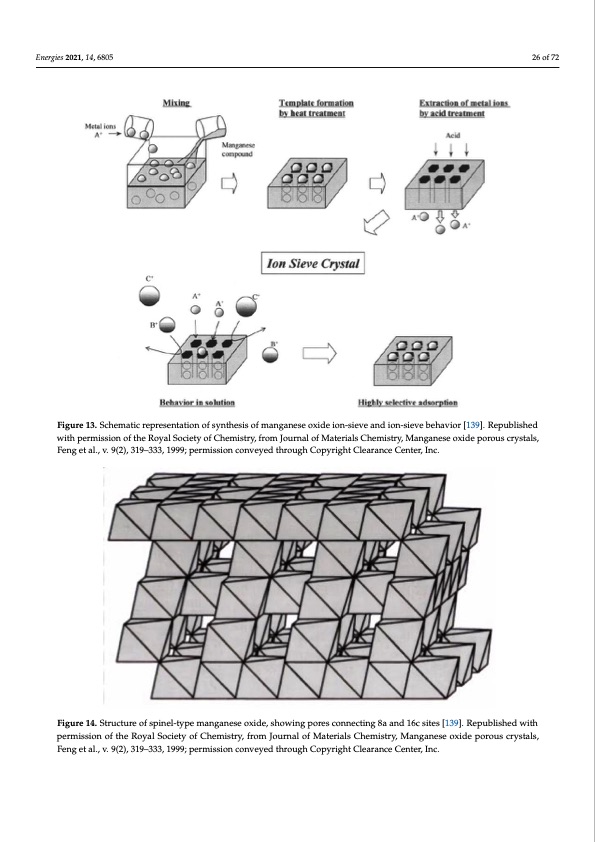
0 used as templates to control their pore dimensions in various synthesis processes (Figure capacity for lithium. The uptake of lithium was positively correlated with increasing Energies 2021, 14, 6805 13) [139]. Manganese oxides show ion-sieve properties and the spinel-type ion-sieves have effective pore radii of approximately 0.7 Å (Figure 14), which makes it selective for adsorption of lithium [139]. Materials that are commonly used as templates for MnOx designed for lithium adsorption include lithium and magnesium; however, other metals may be used [99,101,104,140,141]. 21, 14, x FOR PEER REVIEW 28 of 74 with permission of the Royal Society of Chemistry, from Journal of Materials Chemistry, Manganese oxide porous crystals, with permission of the Royal Society of Chemistry, from Journal of Materials Chemistry, Manganese oxide porous crystals, Feng et al., v. 9(2), 319–333, 1999; permission conveyed through Copyright Clearance Center, Inc. Feng et al., v. 9(2), 319–333, 1999; permission conveyed through Copyright Clearance Center, Inc. 26 of 72 Figure 13. Schematic representation of synthesis of manganese oxide ion-sieve and ion-sieve behavior [139]. Republished Figure 13. Schematic representation of synthesis of manganese oxide ion-sieve and ion-sieve behavior [139]. Republished Figure 14. SFtriugcuturere1o4f.sSptirnuecl-tuyrpe omfasnpgianels-etyopxeidme, ashnogwaninegseporxeisdceo,nsnheocwtinigng8apaonrdes16ccosnitneesc[t1i3n9g].8Raepaunbdlis1h6ecdswiteitsh permission[o1f3t9h]e. RoepyaulbSloischietdy owfiCthepmerismtriys,sfiroonmoJfotuhrenaRloyfaMlaStoecriaeltsyCohfeCmhisetmry,isMtrayn,gfarnoemseJoxuirdneaplorfouMsactreyrsitalls, Fengetal.,vC.h9(e2m),i3s1t9ry–3,3M3,1a9n9g9a;npesrmeisosxioidnecopnovreoyuedstchryoustgahlsC,oFpeynrighettClaela.,ravn.ce9C(2e)n,te3r,1I9n–c3.33, 1999; permission conveyed through Copyright Clearance Center, Inc. Miyai et al. [97] investigated HMnO(Mg) for the sorption of lithium from seawater. They found that HMnO(Mg) was selective for lithium over both monovalent and divalent cations, with a selectivity sequence of Mg < Ca < Sr < Ba < Na = K << Li at pH 8. The MnOx sorbents of composition HMnO(Ca), HMnO(Sr), and HMnO(Ba) had lower selectivity andPDF Image | Recovery of Lithium from Geothermal Brines
PDF Search Title:
Recovery of Lithium from Geothermal BrinesOriginal File Name Searched:
energies-14-06805-v2.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |