
PDF Publication Title:
Text from PDF Page: 002
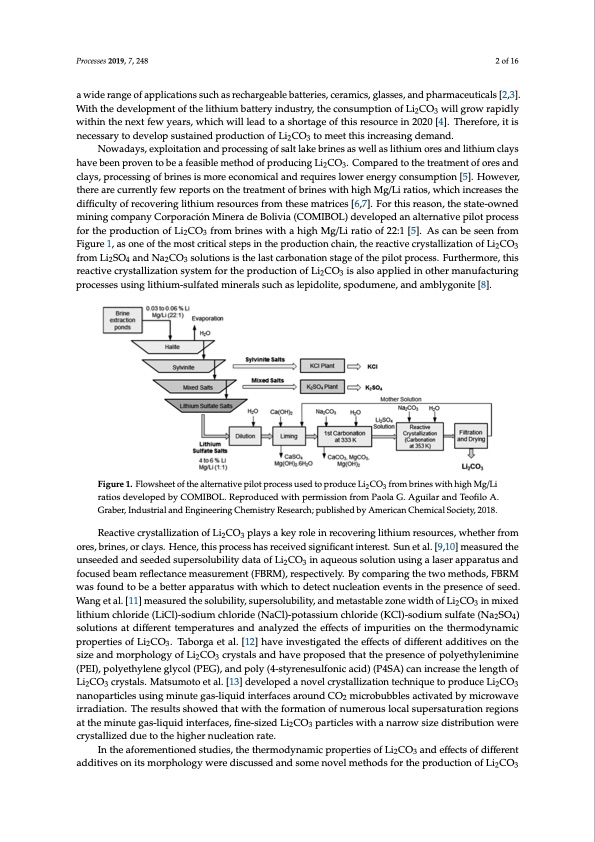
Processes 2019, 7, 248 2 of 16 Processes 2018, 6, x FOR PEER REVIEW 2 of 17 a[2w,3i]d.eWraitnhgethoefadpepvleilcoaptimonesnstuocfhtahserelicthaiurgmeabalettberaytteinridesu,scteryra,mthicesc,ognlassusmesp,atinodnpohfaLrmi2CacOeu3twiciallsg[r2o,3w]. Wraipthidtlhyewdiethvienlothpemneenxttoffewtheyeliatrhsi,uwmhbicahttweriyll ilneaddustotrya,sthoerctaognesuomf tphtiisornesoofuLriceCiOn 20w2i0ll[4g]r.oTwherraepfiodrley, 23 wititshninectehsesanreyxtofedwevyeeloaprss,uwshtaiicnhedwipllroledaudctionaoshfoLri2tCagOe3otoftmhiesertetshoisuirncecrienas2i0n2g0d[4em].aTnhde.refore,itis necesNsaorwyatodadyesv,eelxoplosuitsattaiionedanpdropdruocteisosninogf Lofi2sCaOlt3latokembereitntehsisasinwcrelalsainsglitdheimumanodr.es and lithium claysNhoawveadbaeyesn,epxrpolvoeintationbeanadfeparsoicbelessminegthofosdalotflapkreodburicniensgaLsiw2CeOll3a.sCliotmhiupmareodretsoatnhdeltirtehaiutmecnlatyosf hoarevseabnedenclparyosv,epnrotocebsesianfgeaosfiblreinmeestihsomdorfepercoodnuocminigcaLliaCnOd r.eCquoimrepsalroewdetor ethnertrgeyatcmonesnutmofpotrieosna[n5d]. 23 cHlaoyws,epvreor,cethsseirnegaorfebcruinrresnitslymfoerweerceopnoortmsiocnaltahnedtreaqtumirenstlowf berineneserwgyithcohnisguhmMptgio/Lni[r5a]t.ioHso,wehvicehr, tihnecreasresctuhreredniftfliycufeltwy roefproerctosvoenritnhgeltirtehaiutmernetsofubrcriensefsrowmiththheisgehmMagtr/iLciersa[t6io,7s],.wFohricthisnrcereaasosens, the dstiaffitec-uolwtynoefdrmecionvinergincogmlitphainuymCroerspoouracceisófnroMminthereasedme Baotrlicveisa [(6C,7O].MFIoBrOthLi)sdrevaesloonp,etdheanstatlete-ornwantievde mpiilnoitnpgrococemsspafonrytCheorprordaucicótinonMoinfeLria2CdOe 3Bforloivmiab(rCinOeMs wIBiOthLa) dhiegvhelMopge/dLiarnatailoteorfna22ti:v1e[5p]i.loAtspcraoncebses fsoerenthferopmroFdiguuctrieo1n,aosfoLnieCoOfthferommosbtrcirniteiscawlsithepashinigtheMprgo/Ldiurcatitoionochfa2i2n:,1th[5e].reAacsticvaencbryesstaelelnizafrtoiomn 23 FoifguLri2eC1O,a3sforonmeoLfti2hSeOm4oasntdcriNticaa2ClsOte3psoilnutihoenpsroisduthcetiolnascthcaainrb,tohnearteioanctisvteagcreysotfaltlhizeatpioilnotofpLriocCeOss. 23 fFruormthLeirmSOorea,nthdisNraeaCctOivescorlyustitoanllsiziasttihoenlsayssttceamrbfonratthioenpsrtoadguecotifotnheofpLilio2tCpOro3icseassls.oFuaprtphleiermdoinreo,theisr 24 23 rmeacntuivfaeccturyrsintagllipzraoticoenssseysstuemsinfgorltithheiupmro-dsulcftaitoendomf Lini eCraOls isuaclhso aspplelipeidoinlitoet,hesrpmodaunmufeancet,uraindg 23 pamrobcelyssgeosnuitsein[8g].lithium-sulfated minerals such as lepidolite, spodumene, and amblygonite [8]. Figure 1. Flowsheet of the alternative pilot process used to produce Li CO from brines with high Mg/Li 23 Figure 1. Flowsheet of the alternative pilot process used to produce Li2CO3 from brines with high ratios developed by COMIBOL. Reproduced with permission from Paola G. Aguilar and Teofilo A. Mg/Li ratios developed by COMIBOL. Reproduced with permission from Paola G. Aguilar and Graber, Industrial and Engineering Chemistry Research; published by American Chemical Society, 2018. Teofilo A. Graber, Industrial and Engineering Chemistry Research; published by American Chemical Society, 2018. Reactive crystallization of Li2CO3 plays a key role in recovering lithium resources, whether from ores, brines, or clays. Hence, this process has received significant interest. Sun et al. [9,10] measured the Reactive crystallization of Li2CO3 plays a key role in recovering lithium resources, whether from unseeded and seeded supersolubility data of Li2CO3 in aqueous solution using a laser apparatus and ores, brines, or clays. Hence, this process has received significant interest. Sun et al. [9,10] measured focused beam reflectance measurement (FBRM), respectively. By comparing the two methods, FBRM the unseeded and seeded supersolubility data of Li2CO3 in aqueous solution using a laser apparatus was found to be a better apparatus with which to detect nucleation events in the presence of seed. and focused beam reflectance measurement (FBRM), respectively. By comparing the two methods, Wang et al. [11] measured the solubility, supersolubility, and metastable zone width of Li2CO3 in mixed FBRM was found to be a better apparatus with which to detect nucleation events in the presence of lithium chloride (LiCl)-sodium chloride (NaCl)-potassium chloride (KCl)-sodium sulfate (Na2SO4) seed. Wang et al. [11] measured the solubility, supersolubility, and metastable zone width of Li2CO3 solutions at different temperatures and analyzed the effects of impurities on the thermodynamic in mixed lithium chloride (LiCl)-sodium chloride (NaCl)-potassium chloride (KCl)-sodium sulfate properties of Li2CO3. Taborga et al. [12] have investigated the effects of different additives on the (Na2SO4) solutions at different temperatures and analyzed the effects of impurities on the size and morphology of Li2CO3 crystals and have proposed that the presence of polyethylenimine thermodynamic properties of Li2CO3. Taborga et al. [12] have investigated the effects of different (PEI), polyethylene glycol (PEG), and poly (4-styrenesulfonic acid) (P4SA) can increase the length of additives on the size and morphology of Li2CO3 crystals and have proposed that the presence of Li2CO3 crystals. Matsumoto et al. [13] developed a novel crystallization technique to produce Li2CO3 polyethylenimine (PEI), polyethylene glycol (PEG), and poly (4-styrenesulfonic acid) (P4SA) can nanoparticles using minute gas-liquid interfaces around CO2 microbubbles activated by microwave increase the length of Li2CO3 crystals. Matsumoto et al. [13] developed a novel crystallization irradiation. The results showed that with the formation of numerous local supersaturation regions technique to produce Li2CO3 nanoparticles using minute gas-liquid interfaces around CO2 at the minute gas-liquid interfaces, fine-sized Li2CO3 particles with a narrow size distribution were microbubbles activated by microwave irradiation. The results showed that with the formation of crystallized due to the higher nucleation rate. numerous local supersaturation regions at the minute gas-liquid interfaces, fine-sized Li2CO3 In the aforementioned studies, the thermodynamic properties of Li2CO3 and effects of different particles with a narrow size distribution were crystallized due to the higher nucleation rate. additives on its morphology were discussed and some novel methods for the production of Li2CO3 In the aforementioned studies, the thermodynamic properties of Li2CO3 and effects of different additives on its morphology were discussed and some novel methods for the production of Li2CO3 crystals were also reported [9–13]. However, there have been no works concentrating on the reactivePDF Image | Reactive Crystallization Process of Lithium Carbonate
PDF Search Title:
Reactive Crystallization Process of Lithium CarbonateOriginal File Name Searched:
processes-07-00248-v2.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |