
PDF Publication Title:
Text from PDF Page: 002
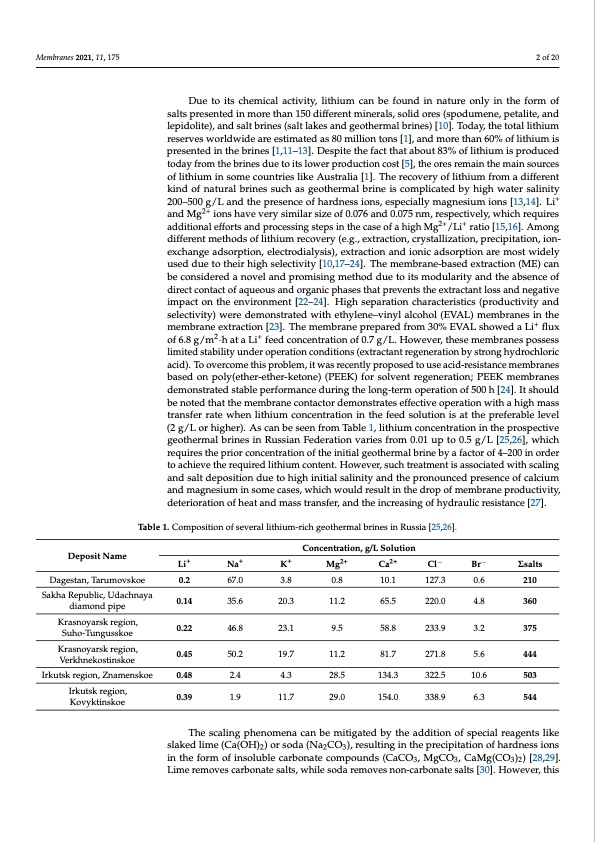
Membranes 2021, 11, 175 2 of 20 Deposit Name Due to its chemical activity, lithium can be found in nature only in the form of salts presented in more than 150 different minerals, solid ores (spodumene, petalite, and lepidolite), and salt brines (salt lakes and geothermal brines) [10]. Today, the total lithium reserves worldwide are estimated as 80 million tons [1], and more than 60% of lithium is presented in the brines [1,11–13]. Despite the fact that about 83% of lithium is produced today from the brines due to its lower production cost [5], the ores remain the main sources of lithium in some countries like Australia [1]. The recovery of lithium from a different kind of natural brines such as geothermal brine is complicated by high water salinity 200–500 g/L and the presence of hardness ions, especially magnesium ions [13,14]. Li+ and Mg2+ ions have very similar size of 0.076 and 0.075 nm, respectively, which requires additional efforts and processing steps in the case of a high Mg2+/Li+ ratio [15,16]. Among different methods of lithium recovery (e.g., extraction, crystallization, precipitation, ion- exchange adsorption, electrodialysis), extraction and ionic adsorption are most widely used due to their high selectivity [10,17–24]. The membrane-based extraction (ME) can be considered a novel and promising method due to its modularity and the absence of direct contact of aqueous and organic phases that prevents the extractant loss and negative impact on the environment [22–24]. High separation characteristics (productivity and selectivity) were demonstrated with ethylene–vinyl alcohol (EVAL) membranes in the membrane extraction [23]. The membrane prepared from 30% EVAL showed a Li+ flux of 6.8 g/m2·h at a Li+ feed concentration of 0.7 g/L. However, these membranes possess limited stability under operation conditions (extractant regeneration by strong hydrochloric acid). To overcome this problem, it was recently proposed to use acid-resistance membranes based on poly(ether-ether-ketone) (PEEK) for solvent regeneration; PEEK membranes demonstrated stable performance during the long-term operation of 500 h [24]. It should be noted that the membrane contactor demonstrates effective operation with a high mass transfer rate when lithium concentration in the feed solution is at the preferable level (2 g/L or higher). As can be seen from Table 1, lithium concentration in the prospective geothermal brines in Russian Federation varies from 0.01 up to 0.5 g/L [25,26], which requires the prior concentration of the initial geothermal brine by a factor of 4–200 in order to achieve the required lithium content. However, such treatment is associated with scaling and salt deposition due to high initial salinity and the pronounced presence of calcium and magnesium in some cases, which would result in the drop of membrane productivity, deterioration of heat and mass transfer, and the increasing of hydraulic resistance [27]. Table 1. Composition of several lithium-rich geothermal brines in Russia [25,26]. Concentration, g/L Solution Li+ Na+ 0.2 67.0 0.14 35.6 0.22 46.8 0.45 50.2 0.48 2.4 0.39 1.9 K+ Mg2+ 3.8 0.8 20.3 11.2 23.1 9.5 19.7 11.2 4.3 28.5 11.7 29.0 Ca2+ Cl− Br− 10.1 127.3 0.6 210 Dagestan, Tarumovskoe Sakha Republic, Udachnaya diamond pipe Krasnoyarsk region, Suho-Tungusskoe Krasnoyarsk region, Verkhnekostinskoe Irkutsk region, Znamenskoe Irkutsk region, Kovyktinskoe Σsalts 65.5 220.0 4.8 360 58.8 233.9 3.2 375 81.7 271.8 5.6 444 134.3 322.5 10.6 503 154.0 338.9 6.3 544 The scaling phenomena can be mitigated by the addition of special reagents like slaked lime (Ca(OH)2) or soda (Na2CO3), resulting in the precipitation of hardness ions in the form of insoluble carbonate compounds (CaCO3, MgCO3, CaMg(CO3)2) [28,29]. Lime removes carbonate salts, while soda removes non-carbonate salts [30]. However, thisPDF Image | Process of Lithium Recovery from Geothermal Brine
PDF Search Title:
Process of Lithium Recovery from Geothermal BrineOriginal File Name Searched:
membranes-11-00175-v2.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |