
PDF Publication Title:
Text from PDF Page: 013
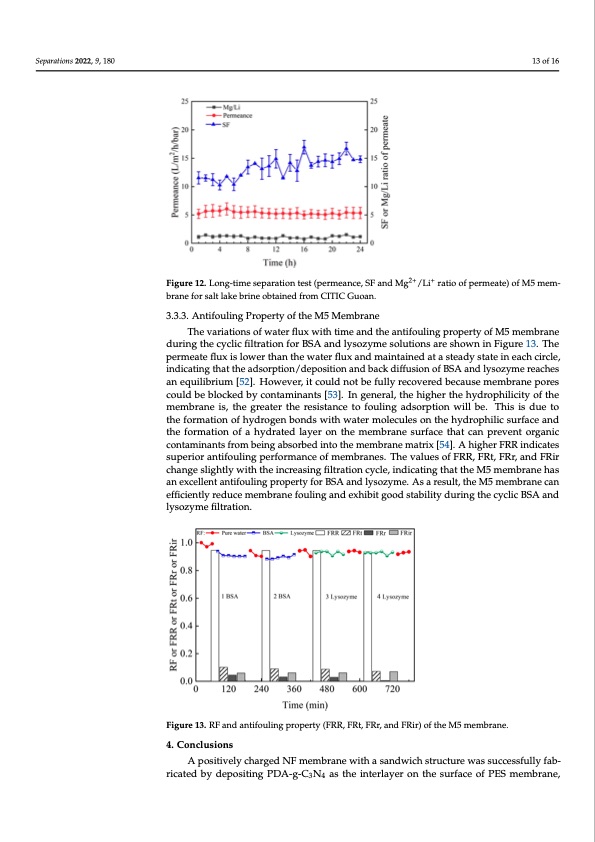
Separations 2022, 9, 180 Table 4 compares the separation performance of several reported NF membranes and M5 membranes prepared in this work. It was found that the rejection of Mg2+ differs sub- stantially among NF membranes. Importantly, the M5 membrane shows higher Mg2+ re- jection and permeance than other NF membranes. According to Figure 12, the permeance (5.01–6.06), Mg2+/Li+ ratio of the permeate (0.73–1.43), and SF (10.28–16.69) change little 13 of 16 during the longtime filtration test. As a result, the M5 membrane has high stability during the filtration process. Separations 2022, 9, x FOR PEER REVIEW 14 of 17 [39] PES/PEI/TMC [49] PES/PEI/TMC [50] (PES-GO)/PEI/TMC [51] Membrane M5 This work PES/PEI/TMC MgCl2 0.4 MPa, 20 °C 0.8 MPa, 25 °C 2.0 g⋅L−1 (salt solution), 21.4 2.0 g⋅L−1 (salt solution), 30 96.9%, 7.13 no report, 12.15 95.6%, 5.80 14 to pure water 4.17 to pure water 3.40 to pure water 2.0 g⋅L−1 (salt solution), 60 −1 2+ + 11.15 to 2.0 g⋅L−1 Figure 12. Long-time separation test (permeance, SF and Mg /Li ratio of permeate) of M5 mem- 0.3FMigPuar,e2152.°CLong-t2im.0egs⋅eLpar(astaioltnstoelsutt(ipoenr)m, 2ea0nce, SF an9d5M.1%2g+,/1L6i+.1r3atio of permeate) of M5 mem- brane for salt lake brine obtained from CITIC Guoan. MgCl2 + LiCl brane for salt lake brine obtained from CITIC Guoan. 3.3.3. Antifouling Property of the M5 Membrane Table 4. Comparison of separation performance of NF membranes in the literature and this work. 3.3.3. Antifouling Property of the M5 Membrane The variations of water flux with time and the antifouling property of M5 membrane The variations of water flux with time and the antifouling property of M5 membrane Operation Rejection Rate of Permeance during the cyclic filtraFteioend SforluBtSiAonand lysozyme solutions are shown in Figure 13. The during the cyclic filtration for BSA and lysozyme solutions are shown in Figure 13. The Conditions Mg2+ and SF (L·m−2·h−1·bar−1) permeate flux is lower than the water flux and maintained at a steady state in each circle, permeate flux is lower than the water flux and maintained at a steady state in each circle, 10.19 to 2.0 g⋅L−1 indicating that the adso−r1ption/deposition and back diffusion of BSA and lysozyme reaches indicatingthatth2e.0adgs⋅Lorp(tsiaolnt/sdoeluptoiosinti)o,n20andbackd98if.f4u%si,o4n8o.0f7BSAandlysozymereaches MgCl2 + LiCl an equilibrium [52]. However, it could not be fully recovered because membrane pores an equilibrium [52]. However, it could not be fully recovered because membrane pores could be blocked by co−1ntaminants [53]. In general, the higher the hydrophilicity of the could be blocked by contaminants [53]. In general, the higher the hydrophilicity of the 2.0 g⋅L (salt solution), 48 93.7%, 12.78 membraneis,thegreatterrttheerreessisistatanncceetotofofuoulilninggadasdosroprtpiotinonwwillilbleb.eT.hiTshiissdiusedtuoethtoe 0.4 MPa, 20 °C 8.66 to 2.0 g⋅L−1 tfhoermfoartmioantiofnhoyfdhryogderon−g1beonnbdosnwdsithwwithatweramterolmecoulleecsuolens tohne thyedhryodprhoilpichisluicrfsaucrefacnedatnhde 2.0 g⋅L (salt solution), 84 92.4%, 10.86 tfhoermfoartmionatoiofnaohfydarhatyedralatyederloanyethreomnethmebmraenmesburrafnaecestuhrafatceanthpartevcaentporegvaennictcoorngtaanmic- cionnantatmsfirnoamntsbefrionmgabesionrgbaebdsionrtboetdhienmtoetmhebmraenmebmratnreixm[5a4tr]i.xA[5h4i]g.hAerhFigRhRerinFdRiRcaitnedsiscuaptes- 3.0 g⋅L−1 (salt lake brine), 27 88.9%, 12.79 sruioprearniotrifoauntlinfoguplienrgfoprmerafonrcmeoafnmceeomfbmraenmebs.rTanhesv.aTluhesvoafluFeRsRo,fFFRRt,RF,RFrR,atl,naFkdeRFrbR,rairncedhaFnRgire −1 −1 0.8csMhliagPnhagt,ley2s5wli°gCithtltyhwe2.i0nthcgrt⋅eLhaesi(nsagclrtfeisalotsrilanutgtiiofinlt)cr,ya2ct0iloe,nincydcilcea,t9in4d.g8i%tcha,at2itn0tg.h0e0thMat5thmeeMm35.b7rmatoneme2.h0bargas⋅nLaenheaxs- acnelelexncetlalennttifaonutliifnogulpinrgoperrotpyerfotyrfBoSrABSaAndanldysloyzsoyzmyem.eA.sAasarerseusultl,t,ththeeM5membranecan e ef ffif ic ciie en nttlly y r re ed du uc ce e m me em mb brra an ne e ffo ou ulliin ng g a an nd d e ex xh hiib biitt g go oo od d s stta ab biilliitty y d du ur riin ng g tth he e c cy yc clliic c B BS SA A a an nd d llysozyme fifillttrattiion.. 8.27 to 2.0 g⋅L−1 MgCl2 + LiCl MgCl2 + LiCl 3.80 to 3.0g⋅L−1 salt Figure 13. RF and antifouling property (FRR, FRt, FRr, and FRir) of the M5 membrane. Figure 13. RF and antifouling property (FRR, FRt, FRr, and FRir) of the M5 membrane. 4. Conclusions 4. Conclusions A positively charged NF membrane with a sandwich structure was successfully fab- A positively charged NF membrane with a sandwich structure was successfully fab- ricated by depositing PDA-g-C N as the interlayer on the surface of PES membrane, ricated by depositing PDA-g-C3N3 4 4as the interlayer on the surface of PES membrane, fol- lowed by the IP process of PEI and TMC on the interlayer. The concentrations of PEI (0.6 wt%), TMC (0.1 wt%), g-C3N4 (0.02 wt%, stripping 96 h), and interlayer reaction time (2 h) are optimized. The final NF membrane (M5) has a low WCA (55.5°) and the IEP is 6.01, which is attributed to the hydrophilic, positively charged active separation layer on the NF membrane surface resulting from the IP process of PEI and TMC. Notably, the perme-PDF Image | Nanofiltration Membrane Using Polydopamine Carbon Nitride
PDF Search Title:
Nanofiltration Membrane Using Polydopamine Carbon NitrideOriginal File Name Searched:
separations-09-00180.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |