
PDF Publication Title:
Text from PDF Page: 002
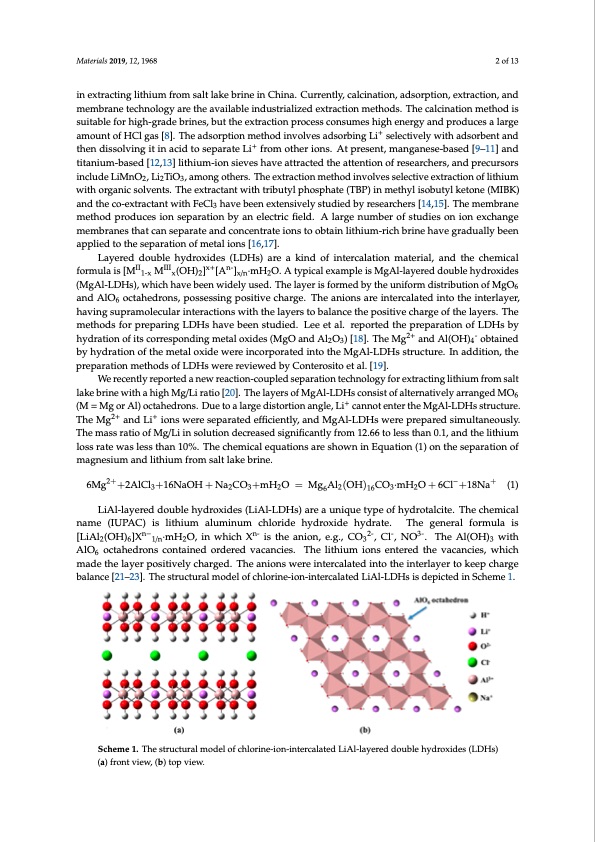
Materials 2019, 12, 1968 2 of 13 in extracting lithium from salt lake brine in China. Currently, calcination, adsorption, extraction, and membrane technology are the available industrialized extraction methods. The calcination method is Materials 2019, 12, x FOR PEER REVIEW 2 of 13 suitable for high-grade brines, but the extraction process consumes high energy and produces a large the high Mg/Li ratio in extracting lithium from salt lake brine in China. Currently, calcination, amount of HCl gas [8]. The adsorption method involves adsorbing Li selectively with adsorbent and adsorption, extraction, and membra+ne technology are the available industrialized extraction then dissolving it in acid to separate Li from other ions. At present, manganese-based [9–11] and methods. The calcination method is suitable for high-grade brines, but the extraction process titanium-based [12,13] lithium-ion sieves have attracted the attention of researchers, and precursors consumes high energy and produces a large amount of HCl gas [8]. The adsorption method involves include LiMnO2, Li2TiO3, among others. The extraction method involves selective extraction of lithium adsorbing Li+ selectively with adsorbent and then dissolving it in acid to separate Li+ from other with organic solvents. The extractant with tributyl phosphate (TBP) in methyl isobutyl ketone (MIBK) ions. At present, manganese-based [9–11] and titanium-based [12,13] lithium-ion sieves have and the co-extractant with FeCl3 have been extensively studied by researchers [14,15]. The membrane attracted the attention of researchers, and precursors include LiMnO2, Li2TiO3, among others. The method produces ion separation by an electric field. A large number of studies on ion exchange extraction method involves selective extraction of lithium with organic solvents. The extractant with membtrribaunteysltphhaotscpahnatsee(pTaBrPa)teinanmdetchoynlciseonbturatytlekioetnosneto(MobIBtaKin)alnitdhituhmec-ori-cehxtrbarcitnaenthwavitehgFreaCdl3uhaallvyebeen appliebdeetnoetxhtenseivpealryasttiuodnieodf mbyerteasleiaornchse[r1s6[,147,]1.5]. The membrane method produces ion separation by an electric field. A large number of studies on ion exchange membranes that can separate and Layered double hydroxides (LDHs) are a kind of intercalation material, and the chemical concentraItIe ionsIItIo obtain lxi+thiumn--rich brine have gradually been applied to the separation of metal formula is [M 1-x M x (OH)2 ] [A ]x/n ·mH2 O. A typical example is MgAl-layered double hydroxides ions [16,17]. (MgAl-LDHs), which have been widely used. The layer is formed by the uniform distribution of MgO6 Layered double hydroxides (LDHs) are a kind of intercalation material, and the chemical and AlO6 octahedrons, possessing positive charge. The anions are intercalated into the interlayer, formula is [MII1-x MIIIx(OH)2]x+[An-]x/n·mH2O. A typical example is MgAl-layered double hydroxides having supramolecular interactions with the layers to balance the positive charge of the layers. The (MgAl-LDHs), which have been widely used. The layer is formed by the uniform distribution of methods for preparing LDHs have been studied. Lee et al. reported the preparation of LDHs by MgO6 and AlO6 octahedrons, possessing positive charge. The anions are intercalated into the hydration of its corresponding metal oxides (MgO and Al O ) [18]. The Mg2+ and Al(OH) - obtained interlayer, having supramolecular interactions with the l2aye3rs to balance the positive charge4of the by hyladyreartsi.oTnhoefmtheethmodestafol ropxriedpeawrinegreLiDnHcosrhpaovreatbedeninstuodtiheed.MLegeAelt-LalD. rHepsosrtreudcthuerep.reInpaaradtdiointionf , the prepaLrDatHiosnbmyheythdoradtsioonfoLfDitsHcsorwresrpeornedviinegwmeedtablyoxCidoenste(MrogsOitoanedtaAl.l2[O193)].[18].TheMg2+andAl(OH)4- obtained by hydration of the metal oxide were incorporated into the MgAl-LDHs structure. In We recently reported a new reaction-coupled separation technology for extracting lithium from salt addition, the preparation methods of LDHs were reviewed by Conterosito et al [19]. lake brine with a high Mg/Li ratio [20]. The layers of MgAl-LDHs consist of alternatively arranged MO6 We recently reported a new reaction-coupled separation technology for extracting lithium from (M = Mg or Al) octahedrons. Due to a large distortion angle, Li+ cannot enter the MgAl-LDHs structure. salt lake brine with a high Mg/Li ratio [20]. The layers of MgAl-LDHs consist of alternatively The Mg2+ and Li+ ions were separated efficiently, and MgAl-LDHs were prepared simultaneously. arranged MO6 (M = Mg or Al) octahedrons. Due to a large distortion angle, Li+ cannot enter the The mass ratio of Mg/Li in solution decreased significantly from 12.66 to less than 0.1, and the lithium MgAl-LDHs structure. The Mg2+ and Li+ ions were separated efficiently, and MgAl-LDHs were loss rate was less than 10%. The chemical equations are shown in Equation (1) on the separation of prepared simultaneously. The mass ratio of Mg/Li in solution decreased significantly from 12.66 to magnesium and lithium from salt lake brine. less than 0.1, and the lithium loss rate was less than 10%. The chemical equations are shown in Equation (1) on the separation of magnesium and lithium from salt lake brine. 6Mg2++2AlCl +16NaOH + Na CO +mH O = Mg Al (OH) CO ·mH O + 6Cl−+18Na+ (1) 32+ 232 621632- + 6Mg +2AlCl3+16NaOH+Na2CO3+mH2O = Mg6Al2OH16CO3·mH2O+6Cl +18Na (1) LiAl-LliaAyle-lraeyderdedoudboluebhleyhdyrdorxoixdiedses(L(LiAiAl-l-LDHs)areauniqiquueetytpyepeofohfyhdyrdotraolctiatlec.iTteh.eTchemchiceamlical namena(mIUePA(IUCP)AiCs)litshiluitmhiumaluamluimnuinmumchclholroirdideehhyyddrroxide hydrraatete..TheThgeengereanlerfaolrmfuolramiusla is n− n− n-n- 2- 2-- 3-- 3- [LiAl2(OH)6]X 1/n·mH2O, in which X is the anion, e.g., CO3 , Cl , NO . The Al(OH)3 with AlO6 [LiAl2 (OH)6 ]X 1/n ·mH2 O, in which X is the anion, e.g., CO3 , Cl , NO . The Al(OH)3 octahedrons contained ordered vacancies. The lithium ions entered the vacancies, which made the with AlO6 octahedrons contained ordered vacancies. The lithium ions entered the vacancies, which layer positively charged. The anions were intercalated into the interlayer to keep charge balance made the layer positively charged. The anions were intercalated into the interlayer to keep charge [21–23]. The structural model of chlorine-ion-intercalated LiAl-LDHs is depicted in Scheme 1. balance [21–23]. The structural model of chlorine-ion-intercalated LiAl-LDHs is depicted in Scheme 1. + Scheme 1. The structural model of chlorine-ion-intercalated LiAl-layered double hydroxides (LDHs) (a) front view, (b) top view.PDF Image | Lithium Recovery Pre-Synthesized Chlorine-Ion-Intercalated
PDF Search Title:
Lithium Recovery Pre-Synthesized Chlorine-Ion-IntercalatedOriginal File Name Searched:
materials-12-01968.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |