
PDF Publication Title:
Text from PDF Page: 015
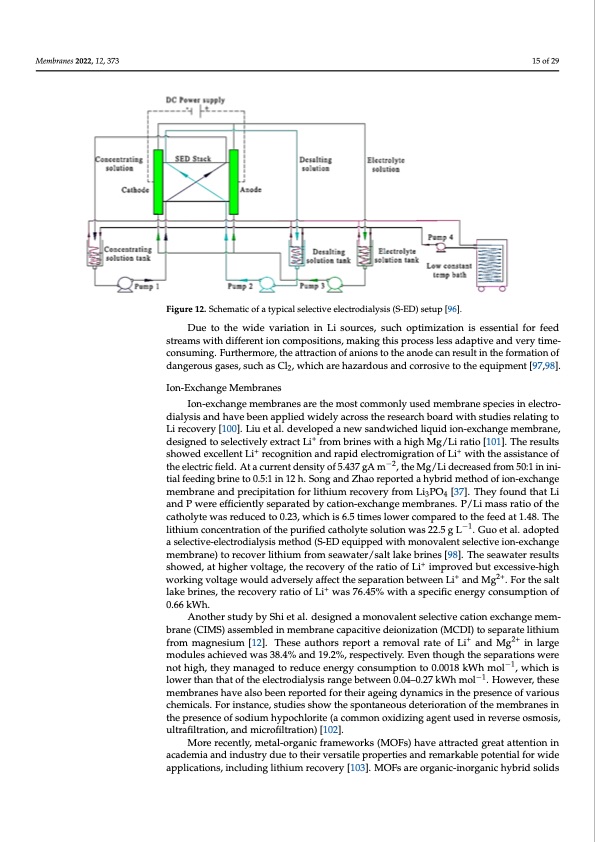
Membranes 2022, 12, 373 a comparable 67.65%:80.08% Li recovery rate [98,99]. Similarly, a 20 °C difference in oper- ating temperature has been shown to improve lithium recovery from 21.47% to 39.2%. Such voltage and temperature tuning are also dependent on specimen compositions. For example, ion composition in the feed has a vital effect on Li recovery efficiency. As re- ported, at elevated operating temperatures, increased recovery rates have been o1b5soefr2v9ed with Mg2+ and Ca2+ ions whilst a decreasing efficiency in the presence of Na+ [96]. FigFuigruer1e21.2S.cShcehmemataitcicooffaatytyppicicaallssellectiveelectrodiiallyssisis(S(S-E-EDD))sesteutupp[9[69]6.]. Due to the wide variation in Li sources, such optimization is essential for feed Due to the wide variation in Li sources, such optimization is essential for feed streams streams with different ion compositions, making this process less adaptive and very time- with different ion compositions, making this process less adaptive and very time-consum- consuming. Furthermore, the attraction of anions to the anode can result in the formation of ing. Furthermore, the attraction of anions to the anode can result in the formation of dan- dangerous gases, such as Cl2, which are hazardous and corrosive to the equipment [97,98]. gerous gases, such as Cl2, which are hazardous and corrosive to the equipment [97,98]. Ion-Exchange Membranes Li recovery [100]. Liu et al. developed a new sandwiched liquid ion-exchange membrane, dialysis and have been applied widely across the research board with studies relating to Ion-Exchange Membranes Ion-exchange membranes are the most commonly used membrane species in electro- dialysis and have been applied widely across the research board with studies relating to Ion-exchange membranes are the most commonly used membrane species in electro- designed to selectively extract Li+ from brines with a high Mg/Li ratio [101]. The results Li recovery [100]. Liu et al. developed a new sandwiched liquid ion-exchange membrane, showed excellent Li+ recognition and rapid electromigration of Li+ with the assistance of designed to selectively extract Li+ from brines with a high Mg/Li ratio [101]. The results the electric field. At a current density of 5.437 gA m−2, the Mg/Li decreased from 50:1 in ini- showed excellent Li+ recognition and rapid electromigration of Li+ with the assistance of tial feeding brine to 0.5:1 in 12 h. Song and Zhao reported a hybrid method of ion-exchange the electric field. At a current density of 5.437 gA m−2, the Mg/Li decreased from 50:1 in membrane and precipitation for lithium recovery from Li3PO4 [37]. They found that Li initial feeding brine to 0.5:1 in 12 h. Song and Zhao reported a hybrid method of ion- and P were efficiently separated by cation-exchange membranes. P/Li mass ratio of the exchange membrane and precipitation for lithium recovery from Li3PO4 [37]. They found catholyte was reduced to 0.23, which is 6.5 times lower compared to the feed at 1.48. The that Li and P were efficiently separated by cation-exchange mem−b1ranes. P/Li mass ratio lithium concentration of the purified catholyte solution was 22.5 g L . Guo et al. adopted Another study by Shi et al. designed a monovalent selective cation exchange mem- brane (CIMS) assembled in membrane capacitive deionization (MCDI) to separate lithium from magnesium [12]. These authors report a removal rate of Li+ and Mg2+ in large modules achieved was 38.4% and 19.2%, respectively. Even though the separations were not high, they managed to reduce energy consumption to 0.0018 kWh mol−1, which is lower than that of the electrodialysis range between 0.04–0.27 kWh mol−1. However, these membranes have also been reported for their ageing dynamics in the presence of various chemicals. For instance, studies show the spontaneous deterioration of the membranes in the presence of sodium hypochlorite (a common oxidizing agent used in reverse osmosis, ultrafiltration, and microfiltration) [102]. More recently, metal-organic frameworks (MOFs) have attracted great attention in academia and industry due to their versatile properties and remarkable potential for wide applications, including lithium recovery [103]. MOFs are organic-inorganic hybrid solids of the catholyte was reduced to 0.23, which is 6.5 times lower compared to the feed at 1.48. a selective-electrodialysis method (S-ED equipped with monovalent selective ion-exchange membrane) to recover lithium from seawater/salt lake brines [98]. The seawater results showed, at higher voltage, the recovery of the ratio of Li+ improved but excessive-high working voltage would adversely affect the separation between Li+ and Mg2+. For the salt lake brines, the recovery ratio of Li+ was 76.45% with a specific energy consumption of 0.66 kWh.PDF Image | Lithium Harvesting using Membranes
PDF Search Title:
Lithium Harvesting using MembranesOriginal File Name Searched:
membranes-12-00373-v2.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |