
PDF Publication Title:
Text from PDF Page: 140
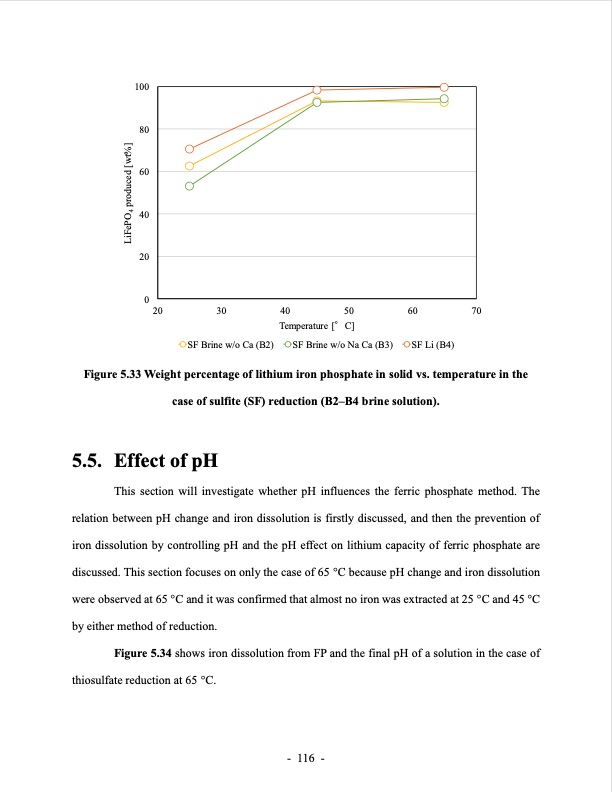
100 80 60 40 20 0 20 30 40 50 60 70 Temperature [°C] SF Brine w/o Ca (B2) SF Brine w/o Na Ca (B3) SF Li (B4) Figure 5.33 Weight percentage of lithium iron phosphate in solid vs. temperature in the case of sulfite (SF) reduction (B2–B4 brine solution). 5.5. Effect of pH This section will investigate whether pH influences the ferric phosphate method. The relation between pH change and iron dissolution is firstly discussed, and then the prevention of iron dissolution by controlling pH and the pH effect on lithium capacity of ferric phosphate are discussed. This section focuses on only the case of 65 °C because pH change and iron dissolution were observed at 65 °C and it was confirmed that almost no iron was extracted at 25 °C and 45 °C by either method of reduction. Figure 5.34 shows iron dissolution from FP and the final pH of a solution in the case of thiosulfate reduction at 65 °C. - 116 - LiFePO4 produced [wt%]PDF Image | LITHIUM EXTRACTION FROM BRINE using ion resin
PDF Search Title:
LITHIUM EXTRACTION FROM BRINE using ion resinOriginal File Name Searched:
3.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |