
PDF Publication Title:
Text from PDF Page: 017
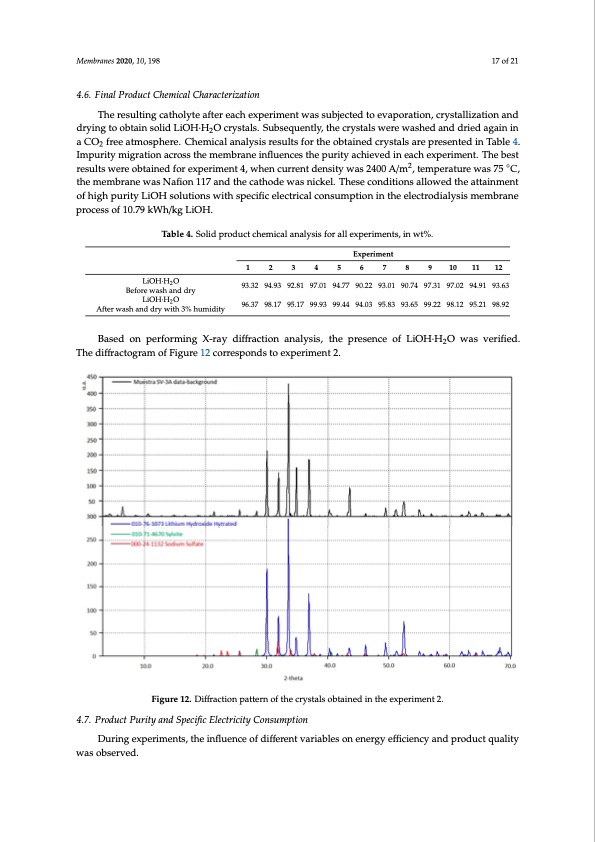
Membranes 2020, 10, 198 17 of 21 4.6. Final Product Chemical Characterization The resulting catholyte after each experiment was subjected to evaporation, crystallization and drying to obtain solid LiOH·H2O crystals. Subsequently, the crystals were washed and dried again in a CO2 free atmosphere. Chemical analysis results for the obtained crystals are presented in Table 4. Membranes 2020, 10, x FOR PEER REVIEW 18 of 22 Impurity migration across the membrane influences the purity achieved in each experiment. The best results were obtained for experiment 4, when current density was 2400 A/m2, temperature was 75 ◦C, Table 4. Solid product chemical analysis for all experiments, in wt%. the membrane was Nafion 117 and the cathode was nickel. These conditions allowed the attainment of high purity LiOH solutions with specific electricalEcxopnesruimpentiton in the electrodialysis membrane processof10.79k1Wh/kgL2iOH. 3 4 5 6 7 8 9 10 11 12 LiOH·H2O Before wash and dry Table 4. Solid product chemical analysis for all experiments, in wt%. 93.32 94.93 92.81 97.01 94.77 90.22 93.01 90.74 97.31 97.02 94.91 93.63 Experiment 1 2 3 4 5 6 7 8 9 10 11 12 93.32 94.93 92.81 97.01 94.77 90.22 93.01 90.74 97.31 97.02 94.91 93.63 LiOH·H2O LiOH·H2 O After wash anBdefore wash and dry 96.37 98.17 95.17 99.93 99.44 94.03 95.83 93.65 99.22 98.12 95.21 98.92 dry with 3% LiOH·H2 O 96.37 98.17 95.17 99.93 99.44 94.03 95.83 93.65 99.22 98.12 95.21 98.92 humidity Based on performing X-ray diffraction analysis, the presence of LiOH·H2O was verified. Based on performing X-ray diffraction analysis, the presence of LiOH∙H2O was verified. The The diffractogram of Figure 12 corresponds to experiment 2. diffractogram of Figure 12 corresponds to experiment 2. Figure 12. Diffraction pattern of the crystals obtained in the experiment 2. Figure 12. Diffraction pattern of the crystals obtained in the experiment 2. 4.7. Product Purity and Specific Electricity Consumption 4.7. Product Purity and Specific Electricity Consumption During experiments, the influence of different variables on energy efficiency and product quality During experiments, the influence of different variables on energy efficiency and product quality After wash and dry with 3% humidity was observed. was observed. Results indicate that product purity was between 93.65 and 99.93%, while specific electrical consumption ranged from 7.25 to 15.24 kWh/kg LiOH at cell current densities between 1200 and 3600 A/m2. In the literature, specific electrical consumption varies between 5 to 15 kWh/kg LiOH at different operating conditions [29], obtaining at best a result of 5 kWh/kg LiOH from a similar initial catholyte concentration (approximately 2.4 wt% LiOH). Our specific electrical consumption results were similar to those previously reported. In relation to the amount of impurities obtained in otherPDF Image | Battery Grade Li Hydroxide by Membrane Electrodialysis
PDF Search Title:
Battery Grade Li Hydroxide by Membrane ElectrodialysisOriginal File Name Searched:
membranes-10-00198.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |