
PDF Publication Title:
Text from PDF Page: 003
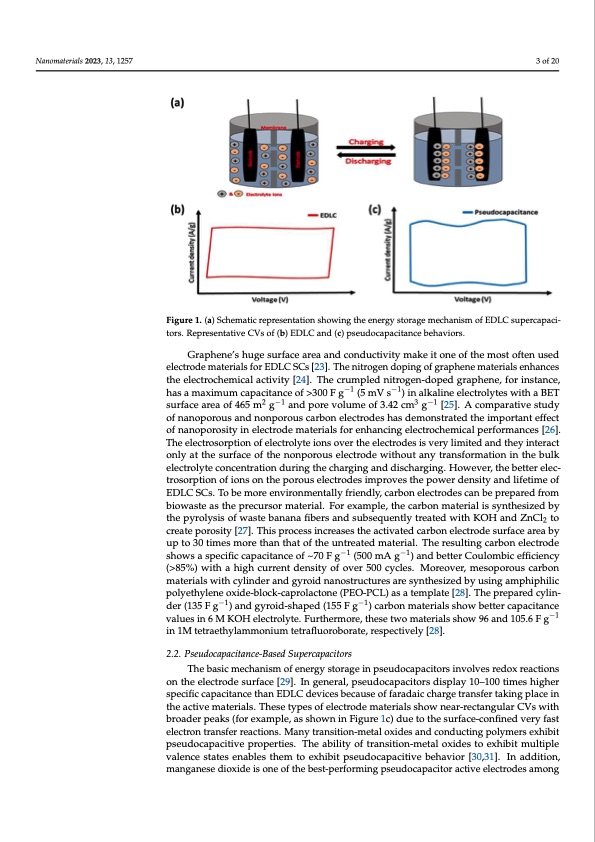
Nanomaterials 2023, 13, x FOR PEER REVIEW Nanomaterials 2023, 13, 1257 3 of 20 Figure 1. (a) Schematic representation showing the energy storage mechanism of EDLC supercapaci- Figure 1. (a) Schematic representation showing the energy storage mechanism of EDLC s tors.RepresentatpivaceitCoVrs.oRfe(pbr)eEseDnLtaCtiavnedC(Vcs)pofse(ubd)oEcDaLpCacaitnadnc(ec)bpesheauvdiorcsa.pacitancebehaviors. Graphene’s huge surface area and conductivity make it one of the most often used Graphene’s huge surface area and conductivity make it one of the most ofte electrode materials for EDLC SCs [23]. The nitrogen doping of graphene materials enhances electrode materials for EDLC SCs [23]. The nitrogen doping of graphene materi the electrochemical activity [24]. The crumpled nitrogen-doped graphene, for instance, hances the electrochemical activity [24]. The crumpled nitrogen-doped graphene, has a maximum capacitance of >300 F g−1 (5 mV s−1) in alkal−i1ne electro−1lytes with a BET stance, has a maximum capacitance of >300 F g (5 mV s ) in alkaline electrolytes surfaceareaof465m2g−1andporevol2um−1eof3.42cm3g−1[25].Acom3p−a1rativestudy BET surface area of 465 m g and pore volume of 3.42 cm g [25]. A comparative of nanoporous and nonporous carbon electrodes has demonstrated the important effect of nanoporous and nonporous carbon electrodes has demonstrated the important of nanoporosity in electrode materials for enhancing electrochemical performances [26]. of nanoporosity in electrode materials for enhancing electrochemical performanc The electrosorption of electrolyte ions over the electrodes is very limited and they interact The electrosorption of electrolyte ions over the electrodes is very limited and they i only at the surface of the nonporous electrode without any transformation in the bulk only at the surface of the nonporous electrode without any transformation in th electrolyte concentration during the charging and discharging. However, the better elec- electrolyte concentration during the charging and discharging. However, the bette trosorption of ions on the porous electrodes improves the power density and lifetime of trosorption of ions on the porous electrodes improves the power density and lifet EDLC SCs. To be more environmentally friendly, carbon electrodes can be prepared from EDLC SCs. To be more environmentally friendly, carbon electrodes can be prepare biowaste as the precursor material. For example, the carbon material is synthesized by biowaste as the precursor material. For example, the carbon material is synthesi the pyrolysis of waste banana fibers and subsequently treated with KOH and ZnCl2 to the pyrolysis of waste banana fibers and subsequently treated with KOH and Z create porosity [27]. This process increases the activated carbon electrode surface area by create porosity [27]. This process increases the activated carbon electrode surface a up to 30 times more than that of the untreated material. The resulting carbon electrode up to 30 times more than that of the untreated material. The resulting carbon ele shows a specific capacitance of ~70 F g−1 (500 mA g−1) and better Coulombic efficiency shows a specific capacitance of ~70 F g−1 (500 mA g−1) and better Coulombic effi (>85%) with a high current density of over 500 cycles. Moreover, mesoporous carbon (>85%) with a high current density of over 500 cycles. Moreover, mesoporous materials with cylinder and gyroid nanostructures are synthesized by using amphiphilic materials with cylinder and gyroid nanostructures are synthesized by using amph polyethylene oxide-block-caprolactone (PEO-PCL) as a template [28]. The prepared cylin- polyethylene oxide-block-caprolactone (PEO-PCL) as a template [28]. The prepar der (135 F g−1) and gyroid-shaped (155 F g−1) carbon materials show better capacitance inder (135 F g−1) and gyroid-shaped (155 F g−1) carbon materials show better capa values in 6 M KOH electrolyte. Furthermore, these two materials show 96 and 105.6 F g−1 values in 6 M KOH electrolyte. Furthermore, these two materials show 96 and 105. in 1M tetraethylammonium tetrafluoroborate, respectively [28]. in 1M tetraethylammonium tetrafluoroborate, respectively [28]. 2.2. Pseudocapacitance-Based Supercapacitors 2.2. Pseudocapacitance-Based Supercapacitors The basic mechanism of energy storage in pseudocapacitors involves redox reactions The basic mechanism of energy storage in pseudocapacitors involves redo on the electrode surface [29]. In general, pseudocapacitors display 10–100 times higher tions on the electrode surface [29]. In general, pseudocapacitors display 10–100 specific capacitance than EDLC devices because of faradaic charge transfer taking place in higher specific capacitance than EDLC devices because of faradaic charge transfer the active materials. These types of electrode materials show near-rectangular CVs with broaderpeaksp(floarcexinamthpelea,catsivsehomwanteirniaFlsig.uTrhee1sec)tdypuestoftheelescutrofadce-mcoantefirniaeldsvsehroywfansetar-recta electron transfeCrVresawctitohnbs.roMaadneyr ptreaankssit(ifoonr-mexeatmalpolxei,daesssahnodwcnoninduFcigtiunrgep1oc)lydmuertsoetxheibsiutrface-co pseudocapacitivveeryprfaospteerlteiecst.roTnhteraanbsifleitryreoafcttriaonsi.tiMona-nmyetralnosxitidoens-mtoeteaxlhoixbidtemsualntidplceonducti valence statesyemnaebrsleesxthiebmit ptoseeuxdhoibcaitppacsietuivdeopcaroppaceirtivees.bTeheavaiboirlit[y30o,3f1t]r.anIsnitaiodnd-imtioetna,l oxides manganese diohxibdiet ims uonlteipolfetvhaelebnecset-psteartfeosrmenianbglepsethuedmoctaopeaxcihtiobritacptsievuedeoleccatproadcietsivaemboenhgavior [30 u n a e n e i d z n c e c x n n ,PDF Image | Water-in-Salt Eutectic Solvent-Based Liquid Electrolytes
PDF Search Title:
Water-in-Salt Eutectic Solvent-Based Liquid ElectrolytesOriginal File Name Searched:
nanomaterials-13-01257.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Power up your energy storage game with Salgenx Salt Water Battery. With its advanced technology, the flow battery provides reliable, scalable, and sustainable energy storage for utility-scale projects. Upgrade to a Salgenx flow battery today and take control of your energy future.
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |