
PDF Publication Title:
Text from PDF Page: 006
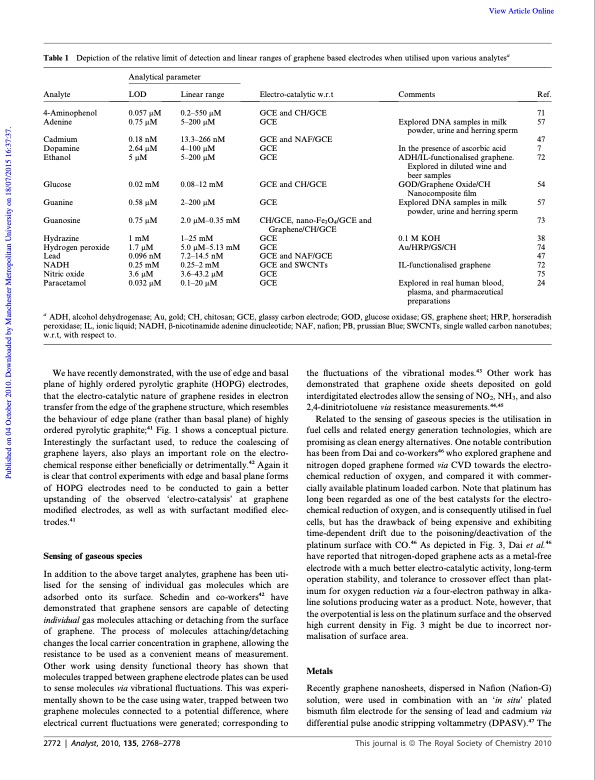
Table 1 Depiction of the relative limit of detection and linear Analytical parameter ranges of graphene based electrodes when utilised upon various analytesa Analyte 4-Aminophenol Adenine Cadmium Dopamine Ethanol Glucose Guanine Guanosine Hydrazine Hydrogen peroxide Lead NADH Nitric oxide Paracetamol LOD 0.057 mM 0.75 mM 0.18 nM 2.64 mM 5mM 0.02 mM 0.58 mM 0.75 mM 1mM 1.7 mM 0.096 nM 0.25 mM 3.6 mM 0.032 mM Linear range 0.2–550 mM 5–200 mM 13.3–266 nM 4–100 mM 5–200 mM 0.08–12 mM 2–200 mM 2.0 mM–0.35 1–25 mM 5.0 mM–5.13 7.2–14.5 nM 0.25–2 mM 3.6–43.2 mM 0.1–20 mM mM mM Electro-catalytic w.r.t GCE and CH/GCE GCE GCE and NAF/GCE GCE GCE GCE and CH/GCE GCE CH/GCE, nano-Fe3O4/GCE and Graphene/CH/GCE GCE GCE GCE and NAF/GCE GCE and SWCNTs GCE GCE Comments Ref. 71 Explored DNA samples in milk 57 powder, urine and herring sperm 47 In the presence of ascorbic acid 7 ADH/IL-functionalised graphene. 72 Explored in diluted wine and beer samples GOD/Graphene Oxide/CH 54 Nanocomposite film Explored DNA samples in milk 57 powder, urine and herring sperm 73 0.1 M KOH 38 Au/HRP/GS/CH 74 47 IL-functionalised graphene 72 75 Explored in real human blood, 24 plasma, and pharmaceutical preparations a ADH, alcohol dehydrogenase; Au, gold; CH, chitosan; GCE, glassy carbon electrode; GOD, glucose oxidase; GS, graphene sheet; HRP, horseradish peroxidase; IL, ionic liquid; NADH, b-nicotinamide adenine dinucleotide; NAF, nafion; PB, prussian Blue; SWCNTs, single walled carbon nanotubes; w.r.t, with respect to. We have recently demonstrated, with the use of edge and basal plane of highly ordered pyrolytic graphite (HOPG) electrodes, that the electro-catalytic nature of graphene resides in electron transfer from the edge of the graphene structure, which resembles the behaviour of edge plane (rather than basal plane) of highly ordered pyrolytic graphite;41 Fig. 1 shows a conceptual picture. Interestingly the surfactant used, to reduce the coalescing of graphene layers, also plays an important role on the electro- chemical response either beneficially or detrimentally.42 Again it is clear that control experiments with edge and basal plane forms of HOPG electrodes need to be conducted to gain a better upstanding of the observed ‘electro-catalysis’ at graphene modified electrodes, as well as with surfactant modified elec- trodes.41 Sensing of gaseous species In addition to the above target analytes, graphene has been uti- lised for the sensing of individual gas molecules which are adsorbed onto its surface. Schedin and co-workers42 have demonstrated that graphene sensors are capable of detecting individual gas molecules attaching or detaching from the surface of graphene. The process of molecules attaching/detaching changes the local carrier concentration in graphene, allowing the resistance to be used as a convenient means of measurement. Other work using density functional theory has shown that molecules trapped between graphene electrode plates can be used to sense molecules via vibrational fluctuations. This was experi- mentally shown to be the case using water, trapped between two graphene molecules connected to a potential difference, where electrical current fluctuations were generated; corresponding to the fluctuations of the vibrational modes.43 Other work has demonstrated that graphene oxide sheets deposited on gold interdigitated electrodes allow the sensing of NO2, NH3, and also 2,4-dinitriotoluene via resistance measurements.44,45 Related to the sensing of gaseous species is the utilisation in fuel cells and related energy generation technologies, which are promising as clean energy alternatives. One notable contribution has been from Dai and co-workers46 who explored graphene and nitrogen doped graphene formed via CVD towards the electro- chemical reduction of oxygen, and compared it with commer- cially available platinum loaded carbon. Note that platinum has long been regarded as one of the best catalysts for the electro- chemical reduction of oxygen, and is consequently utilised in fuel cells, but has the drawback of being expensive and exhibiting time-dependent drift due to the poisoning/deactivation of the platinum surface with CO.46 As depicted in Fig. 3, Dai et al.46 have reported that nitrogen-doped graphene acts as a metal-free electrode with a much better electro-catalytic activity, long-term operation stability, and tolerance to crossover effect than plat- inum for oxygen reduction via a four-electron pathway in alka- line solutions producing water as a product. Note, however, that the overpotential is less on the platinum surface and the observed high current density in Fig. 3 might be due to incorrect nor- malisation of surface area. Metals Recently graphene nanosheets, dispersed in Nafion (Nafion-G) solution, were used in combination with an ‘in situ’ plated bismuth film electrode for the sensing of lead and cadmium via differential pulse anodic stripping voltammetry (DPASV).47 The 2772 | Analyst, 2010, 135, 2768–2778 This journal is a The Royal Society of Chemistry 2010 View Article Online Published on 04 October 2010. Downloaded by Manchester Metropolitan University on 18/07/2015 16:37:37.PDF Image | Graphene Electrochemistry
PDF Search Title:
Graphene ElectrochemistryOriginal File Name Searched:
c0an00590h.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Power up your energy storage game with Salgenx Salt Water Battery. With its advanced technology, the flow battery provides reliable, scalable, and sustainable energy storage for utility-scale projects. Upgrade to a Salgenx flow battery today and take control of your energy future.
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |