PDF Publication Title:
Text from PDF Page: 008
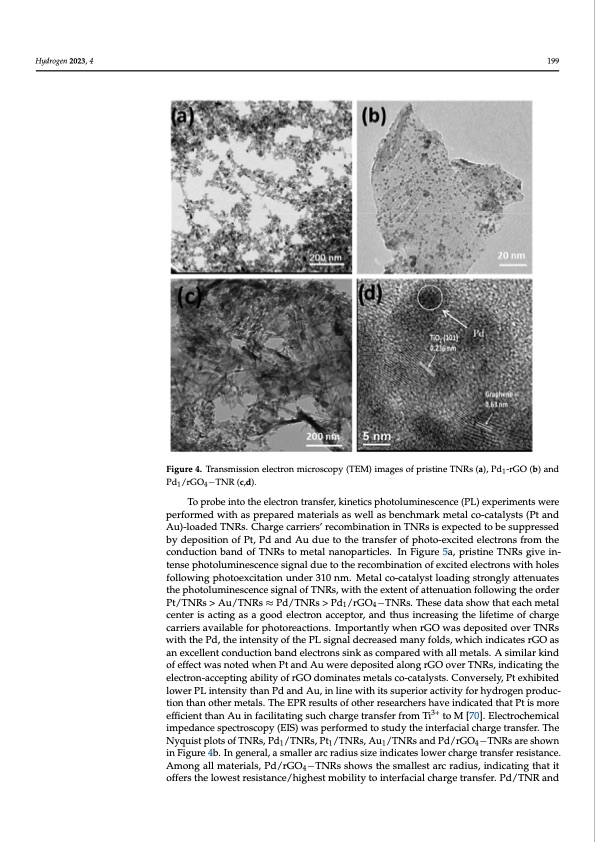
Hydrogen 2023, 4 Figure 3. XPS C1s (a) and XPS Pd3d (b) spectra of Pd1/rGO4−TNR. The inset in (a) is a rescaled (a) 199 to clearly show XPS C1s peaks with a relatively lower intensity. Figure 4. Transmission electron microscopy (TEM) images of pristine TNRs (a), Pd1-rGO (b) and Figure 4. Transmission electron microscopy (TEM) images of pristine TNRs (a), Pd -rGO (b) and Pd1/rGO4−TNR (c,d). Pd1/rGO4−TNR (c,d). 1 To probe into the electron transfer, kinetics photoluminescence (PL) experiments To probe into the electron transfer, kinetics photoluminescence (PL) experiments were were performed with as prepared materials as well as benchmark metal co-catalysts (Pt performed with as prepared materials as well as benchmark metal co-catalysts (Pt and Au)-loaded TNRs. Charge carriers’ recombination in TNRs is expected to be suppressed by deposition of Pt, Pd and Au due to the transfer of photo-excited electrons from the conduction band of TNRs to metal nanoparticles. In Figure 5a, pristine TNRs give in- tense photoluminescence signal due to the recombination of excited electrons with holes following photoexcitation under 310 nm. Metal co-catalyst loading strongly attenuates the photoluminescence signal of TNRs, with the extent of attenuation following the order Pt/TNRs > Au/TNRs ≈ Pd/TNRs > Pd1/rGO4−TNRs. These data show that each metal center is acting as a good electron acceptor, and thus increasing the lifetime of charge carriers available for photoreactions. Importantly when rGO was deposited over TNRs with the Pd, the intensity of the PL signal decreased many folds, which indicates rGO as an excellent conduction band electrons sink as compared with all metals. A similar kind of effect was noted when Pt and Au were deposited along rGO over TNRs, indicating the electron-accepting ability of rGO dominates metals co-catalysts. Conversely, Pt exhibited lower PL intensity than Pd and Au, in line with its superior activity for hydrogen produc- tion than other metals. The EPR results of other researchers have indicated that Pt is more efficient than Au in facilitating such charge transfer from Ti3+ to M [70]. Electrochemical impedance spectroscopy (EIS) was performed to study the interfacial charge transfer. The Nyquist plots of TNRs, Pd1/TNRs, Pt1/TNRs, Au1/TNRs and Pd/rGO4−TNRs are shown in Figure 4b. In general, a smaller arc radius size indicates lower charge transfer resistance. Among all materials, Pd/rGO4−TNRs shows the smallest arc radius, indicating that it offers the lowest resistance/highest mobility to interfacial charge transfer. Pd/TNR andPDF Image | Enhanced Photoreforming of Oxygenates
PDF Search Title:
Enhanced Photoreforming of OxygenatesOriginal File Name Searched:
hydrogen-04-00014-v2.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Power up your energy storage game with Salgenx Salt Water Battery. With its advanced technology, the flow battery provides reliable, scalable, and sustainable energy storage for utility-scale projects. Upgrade to a Salgenx flow battery today and take control of your energy future.
CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com (Standard Web Page)