
PDF Publication Title:
Text from PDF Page: 002
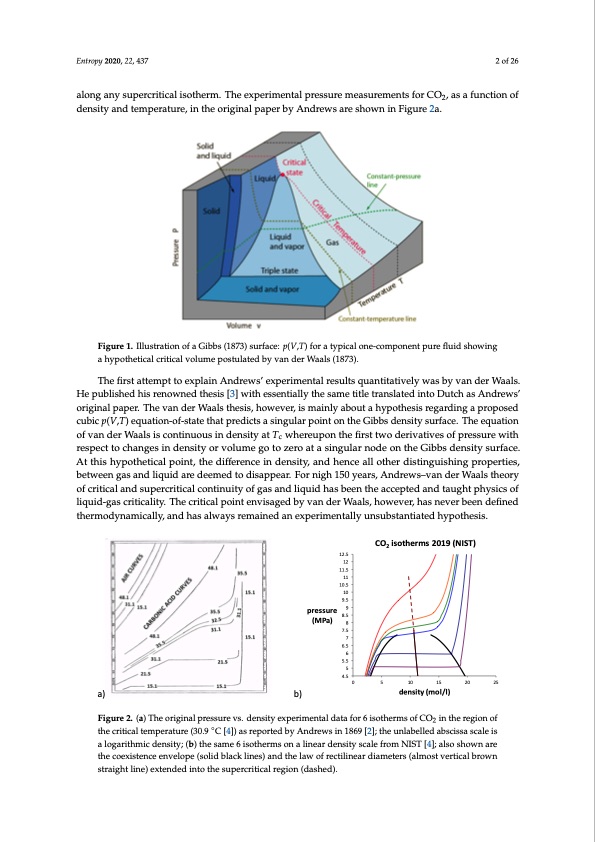
He published his renowned thesis [3] with essentially the same title translated into Dutch as Andrews’ original paper. The van der Waals thesis, however, is mainly about a hypothesis regarding a proposed cubic p(V,T) equation-of-state that predicts a singular point on the Gibbs density surface. The equation of van der Waals is continuous in density at Tc whereupon the first two derivatives of pressure with respect to changes in density or volume go to zero at a singular node on the Gibbs density surface. At Entropy 2020, 22, 437 2 of 26 this hypothetical point, the difference in density, and hence all other distinguishing properties, between gas and liquid are deemed to disappear. For nigh 150 years, Andrews–van der Waals theory of critical and supercritical continuity of gas and liquid has been the accepted and taught physics of along any supercritical isotherm. The experimental pressure measurements for CO2, as a function of liquid-gas criticality. The critical point envisaged by van der Waals, however, has never been defined density and temperature, in the original paper by Andrews are shown in Figure 2a. thermodynamically, and has always remained an experimentally unsubstantiated hypothesis. 2 of 27 along any supercritical isotherm. The experimental pressure measurements for CO2, as a function of density and temperature, in the original paper by Andrews are shown in Figure 2a. The first attempt to explain Andrews’ experimental results quantitatively was by van der Waals. He published his renowned thesis [3] with essentially the same title translated into Dutch as Andrews’ original paper. The van der Waals thesis, however, is mainly about a hypothesis regarding a proposed cubic p(V,T) equation-of-state that predicts a singular point on the Gibbs density surface. The equation of van der Waals is continuous in density at Tc whereupon the first two derivatives of pressure with respect to changes in density or volume go to zero at a singular node on the Gibbs density surface. At this hypothetical point, the difference in density, and hence all other distinguishing properties, between gas and liquid are deemed to disappear. For nigh 150 years, Andrews–van der Waals theory of critical and supercritical continuity of gas and liquid has been the accepted and taught physics of liquid-gas criticality. The critical point envisaged by van der Waals, however, has never been defined thermodynamically, and has always remained an experimentally unsubstantiated hypothesis. Figure 1. Illustration of a Gibbs (1873) surface: p(V,T) for a typical one-component pure fluid showing Figure 1. Illustration of a Gibbs (1873) surface: p(V,T) for a typical one-component pure fluid showing a a hypothetical critical volume postulated by van der Waals (1873). hypothetical critical volume postulated by van der Waals (1873). The first attempt to explain Andrews’ experimental results quantitatively was by van der Waals. CO 12 11.5 of van der Waals is continuous in density at Tc whereupon th10e.5 first two derivatives of pressure with 10 of critical and supercritical continuity of gas and liquid has been the accepted and taught physics of He published his renowned thesis [3] with essentially the same title translated into Dutch as Andrews’ 12.5 original paper. The van der Waals thesis, however, is mainly about a hypothesis regarding a proposed cubic p(V,T) equation-of-state that predicts a singular point on the Gibbs density surface. The equation 11 respect to changes in density or volume go to zero at a singular node on the Gibbs density surface. 9.5 At this hypothetical point, the difference in density, and hen9ce all other distinguishing properties, pressure 8.5 between gas and liquid are deemed to disappear. For (nMigPha)1508years, Andrews–van der Waals theory 7 liquid-gas criticality. The critical point envisaged by van der W6.5aals, however, has never been defined Figure 1. Illustration of a Gibbs (1873) surface: p(V,T) for a typical6one-component pure fluid showing a 5.5 5 4.5 a) b) Figure 2. (a) The original pressure vs. density experim the critical temperature (30.9 °C [4]) as reported by An alogarithmicdensity;(b)thesame6isothermsonale a) b) thermodynamically, and has always remained an experimentally unsubstantiated hypothesis. hypothetical critical volume postulated by van der Waals (1873). 7.5 2 isotherms 2019 (NIST) pressure 9 12.5 12 11.5 11 10.5 CO isotherms2019(NIST) 0 25 10 15 20 density (mol/l) ental dat10a for 6 isotherms of CO2 in the region o 9.5 drews in 1869 [2]; the unlabelled abscissa scale i inear density scale from NIST [4]; also shown ar (MPa) 8.5 8 7.5 7 6.5 6 5.5 5 4.5 0 5 10 15 20 25 density (mol/l) Figure 2. (a) The original pressure vs. density experimental data for 6 isotherms of CO2 in the region of Figure 2. (a) The original pressure vs. density experimental data for 6 isotherms of CO2 in the region of the critical temperature (30.9 ◦C [4]) as reported by Andrews in 1869 [2]; the unlabelled abscissa scale is the critical temperature (30.9 °C [4]) as reported by Andrews in 1869 [2]; the unlabelled abscissa scale is a logarithmic density; (b) the same 6 isotherms on a linear density scale from NIST [4]; also shown are a logarithmic density; (b) the same 6 isotherms on a linear density scale from NIST [4]; also shown are the coexistence envelope (solid black lines) and the law of rectilinear diameters (almost vertical brown straight line) extended into the supercritical region (dashed). f s 25PDF Image | Supercritical Fluid Gaseous and Liquid States
PDF Search Title:
Supercritical Fluid Gaseous and Liquid StatesOriginal File Name Searched:
entropy-22-00437.pdfDIY PDF Search: Google It | Yahoo | Bing
Sulfur Deposition on Carbon Nanofibers using Supercritical CO2 Sulfur Deposition on Carbon Nanofibers using Supercritical CO2. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur... More Info
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |