
PDF Publication Title:
Text from PDF Page: 003
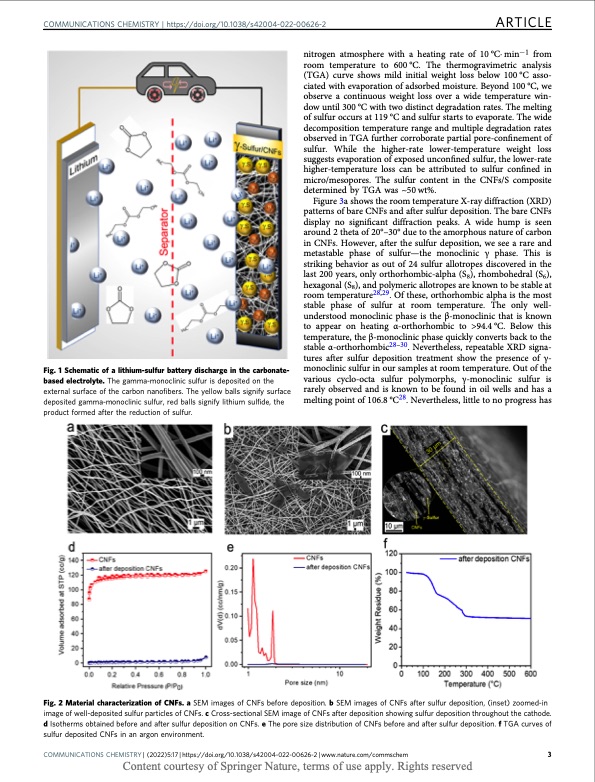
COMMUNICATIONS CHEMISTRY | https://doi.org/10.1038/s42004-022-00626-2 ARTICLE Fig. 1 Schematic of a lithium-sulfur battery discharge in the carbonate- based electrolyte. The gamma-monoclinic sulfur is deposited on the external surface of the carbon nanofibers. The yellow balls signify surface deposited gamma-monoclinic sulfur, red balls signify lithium sulfide, the product formed after the reduction of sulfur. nitrogen atmosphere with a heating rate of 10°C·min−1 from room temperature to 600°C. The thermogravimetric analysis (TGA) curve shows mild initial weight loss below 100 °C asso- ciated with evaporation of adsorbed moisture. Beyond 100 °C, we observe a continuous weight loss over a wide temperature win- dow until 300 °C with two distinct degradation rates. The melting of sulfur occurs at 119 °C and sulfur starts to evaporate. The wide decomposition temperature range and multiple degradation rates observed in TGA further corroborate partial pore-confinement of sulfur. While the higher-rate lower-temperature weight loss suggests evaporation of exposed unconfined sulfur, the lower-rate higher-temperature loss can be attributed to sulfur confined in micro/mesopores. The sulfur content in the CNFs/S composite determined by TGA was ~50 wt%. Figure 3a shows the room temperature X-ray diffraction (XRD) patterns of bare CNFs and after sulfur deposition. The bare CNFs display no significant diffraction peaks. A wide hump is seen around 2 theta of 20°–30° due to the amorphous nature of carbon in CNFs. However, after the sulfur deposition, we see a rare and metastable phase of sulfur—the monoclinic γ phase. This is striking behavior as out of 24 sulfur allotropes discovered in the last 200 years, only orthorhombic-alpha (S8), rhombohedral (S6), hexagonal (S8), and polymeric allotropes are known to be stable at room temperature28,29. Of these, orthorhombic alpha is the most stable phase of sulfur at room temperature. The only well- understood monoclinic phase is the β-monoclinic that is known to appear on heating α-orthorhombic to >94.4 °C. Below this temperature, the β-monoclinic phase quickly converts back to the stable α-orthorhombic28–30. Nevertheless, repeatable XRD signa- tures after sulfur deposition treatment show the presence of γ- monoclinic sulfur in our samples at room temperature. Out of the various cyclo-octa sulfur polymorphs, γ-monoclinic sulfur is rarely observed and is known to be found in oil wells and has a melting point of 106.8 °C28. Nevertheless, little to no progress has Fig. 2 Material characterization of CNFs. a SEM images of CNFs before deposition. b SEM images of CNFs after sulfur deposition, (inset) zoomed-in image of well-deposited sulfur particles of CNFs. c Cross-sectional SEM image of CNFs after deposition showing sulfur deposition throughout the cathode. d Isotherms obtained before and after sulfur deposition on CNFs. e The pore size distribution of CNFs before and after sulfur deposition. f TGA curves of sulfur deposited CNFs in an argon environment. COMMUNICATIONS CHEMISTRY | (2022)5:17 | https://doi.org/10.1038/s42004-022-00626-2 | www.nature.com/commschem 3 Content courtesy of Springer Nature, terms of use apply. Rights reservedPDF Image | Stabilization of gamma sulfur at room temperature
PDF Search Title:
Stabilization of gamma sulfur at room temperatureOriginal File Name Searched:
Stabilization_of_gamma_sulfur_at_room_temperature_.pdfDIY PDF Search: Google It | Yahoo | Bing
Sulfur Deposition on Carbon Nanofibers using Supercritical CO2 Sulfur Deposition on Carbon Nanofibers using Supercritical CO2. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur... More Info
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |