
PDF Publication Title:
Text from PDF Page: 131
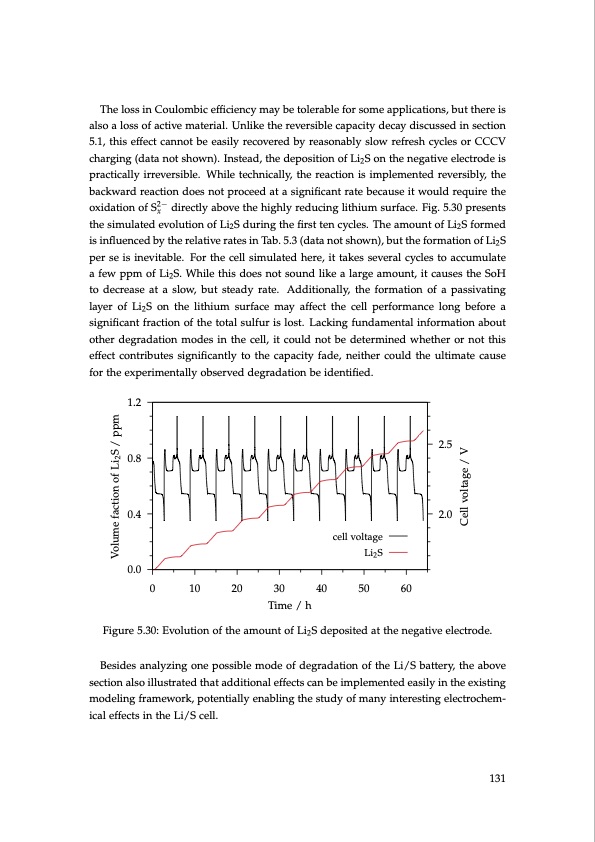
The loss in Coulombic efficiency may be tolerable for some applications, but there is also a loss of active material. Unlike the reversible capacity decay discussed in section 5.1, this effect cannot be easily recovered by reasonably slow refresh cycles or CCCV charging (data not shown). Instead, the deposition of Li2S on the negative electrode is practically irreversible. While technically, the reaction is implemented reversibly, the backward reaction does not proceed at a significant rate because it would require the oxidation of S2− directly above the highly reducing lithium surface. Fig. 5.30 presents x the simulated evolution of Li2S during the first ten cycles. The amount of Li2S formed is influenced by the relative rates in Tab. 5.3 (data not shown), but the formation of Li2S per se is inevitable. For the cell simulated here, it takes several cycles to accumulate a few ppm of Li2S. While this does not sound like a large amount, it causes the SoH to decrease at a slow, but steady rate. Additionally, the formation of a passivating layer of Li2S on the lithium surface may affect the cell performance long before a significant fraction of the total sulfur is lost. Lacking fundamental information about other degradation modes in the cell, it could not be determined whether or not this effect contributes significantly to the capacity fade, neither could the ultimate cause for the experimentally observed degradation be identified. 1.2 0.8 0.4 2.5 2.0 cell voltage Li2 S Volume faction of Li2S / ppm Cell voltage / V 0.0 0 10 20 30 40 50 60 Time / h Figure 5.30: Evolution of the amount of Li2S deposited at the negative electrode. Besides analyzing one possible mode of degradation of the Li/S battery, the above section also illustrated that additional effects can be implemented easily in the existing modeling framework, potentially enabling the study of many interesting electrochem- ical effects in the Li/S cell. 131PDF Image | Lithium-Sulfur Battery: Design, Characterization, and Physically-based Modeling
PDF Search Title:
Lithium-Sulfur Battery: Design, Characterization, and Physically-based ModelingOriginal File Name Searched:
Dissertation_David_N._Fronczek_The_Lithium_Sulfur_Battery.pdfDIY PDF Search: Google It | Yahoo | Bing
Sulfur Deposition on Carbon Nanofibers using Supercritical CO2 Sulfur Deposition on Carbon Nanofibers using Supercritical CO2. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur... More Info
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |