
PDF Publication Title:
Text from PDF Page: 129
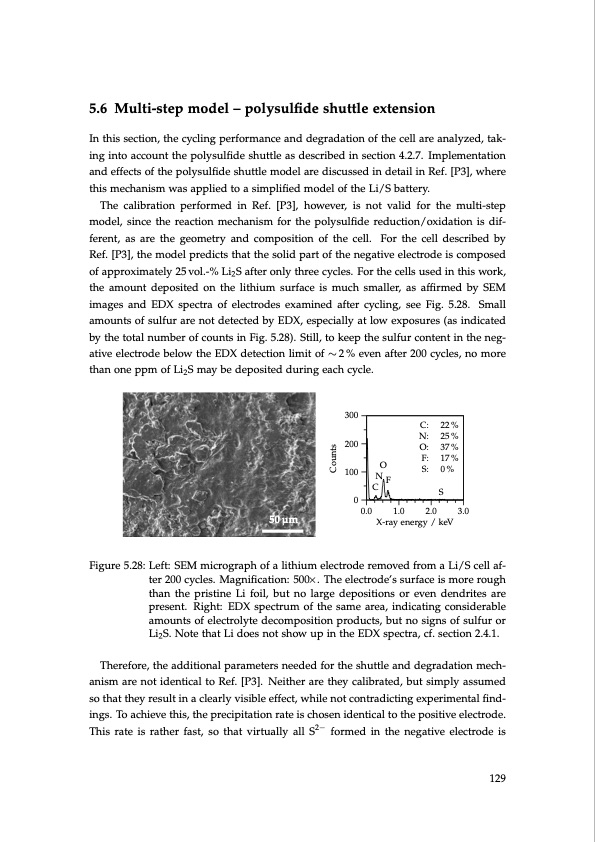
5.6 Multi-step model – polysulfide shuttle extension In this section, the cycling performance and degradation of the cell are analyzed, tak- ing into account the polysulfide shuttle as described in section 4.2.7. Implementation and effects of the polysulfide shuttle model are discussed in detail in Ref. [P3], where this mechanism was applied to a simplified model of the Li/S battery. The calibration performed in Ref. [P3], however, is not valid for the multi-step model, since the reaction mechanism for the polysulfide reduction/oxidation is dif- ferent, as are the geometry and composition of the cell. For the cell described by Ref. [P3], the model predicts that the solid part of the negative electrode is composed of approximately 25 vol.-% Li2S after only three cycles. For the cells used in this work, the amount deposited on the lithium surface is much smaller, as affirmed by SEM images and EDX spectra of electrodes examined after cycling, see Fig. 5.28. Small amounts of sulfur are not detected by EDX, especially at low exposures (as indicated by the total number of counts in Fig. 5.28). Still, to keep the sulfur content in the neg- ative electrode below the EDX detection limit of ∼ 2 % even after 200 cycles, no more than one ppm of Li2S may be deposited during each cycle. 50 μm 0.0 1.0 2.0 3.0 Figure 5.28: Left: SEM micrograph of a lithium electrode removed from a Li/S cell af- ter 200 cycles. Magnification: 500×. The electrode’s surface is more rough than the pristine Li foil, but no large depositions or even dendrites are present. Right: EDX spectrum of the same area, indicating considerable amounts of electrolyte decomposition products, but no signs of sulfur or Li2S. Note that Li does not show up in the EDX spectra, cf. section 2.4.1. Therefore, the additional parameters needed for the shuttle and degradation mech- anism are not identical to Ref. [P3]. Neither are they calibrated, but simply assumed so that they result in a clearly visible effect, while not contradicting experimental find- ings. To achieve this, the precipitation rate is chosen identical to the positive electrode. This rate is rather fast, so that virtually all S2− formed in the negative electrode is 300 200 100 C S C: 22% N: 25% O: 37% F: 17% S: 0% NF O 0 X-ray energy / keV 129 CountsPDF Image | Lithium-Sulfur Battery: Design, Characterization, and Physically-based Modeling
PDF Search Title:
Lithium-Sulfur Battery: Design, Characterization, and Physically-based ModelingOriginal File Name Searched:
Dissertation_David_N._Fronczek_The_Lithium_Sulfur_Battery.pdfDIY PDF Search: Google It | Yahoo | Bing
Sulfur Deposition on Carbon Nanofibers using Supercritical CO2 Sulfur Deposition on Carbon Nanofibers using Supercritical CO2. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur... More Info
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |