
PDF Publication Title:
Text from PDF Page: 419
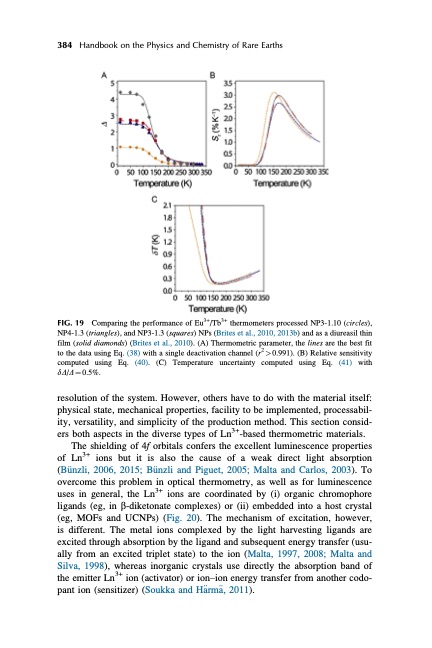
384 Handbook on the Physics and Chemistry of Rare Earths FIG. 19 Comparing the performance of Eu3+/Tb3+ thermometers processed NP3-1.10 (circles), NP4-1.3 (triangles), and NP3-1.3 (squares) NPs (Brites et al., 2010, 2013b) and as a diureasil thin film (solid diamonds) (Brites et al., 2010). (A) Thermometric parameter, the lines are the best fit to the data using Eq. (38) with a single deactivation channel (r2 > 0.991). (B) Relative sensitivity computed using Eq. (40). (C) Temperature uncertainty computed using Eq. (41) with dD/D 1⁄4 0.5%. resolution of the system. However, others have to do with the material itself: physical state, mechanical properties, facility to be implemented, processabil- ity, versatility, and simplicity of the production method. This section consid- ers both aspects in the diverse types of Ln3+-based thermometric materials. The shielding of 4f orbitals confers the excellent luminescence properties of Ln3+ ions but it is also the cause of a weak direct light absorption (B€unzli, 2006, 2015; B€unzli and Piguet, 2005; Malta and Carlos, 2003). To overcome this problem in optical thermometry, as well as for luminescence uses in general, the Ln3+ ions are coordinated by (i) organic chromophore ligands (eg, in b-diketonate complexes) or (ii) embedded into a host crystal (eg, MOFs and UCNPs) (Fig. 20). The mechanism of excitation, however, is different. The metal ions complexed by the light harvesting ligands are excited through absorption by the ligand and subsequent energy transfer (usu- ally from an excited triplet state) to the ion (Malta, 1997, 2008; Malta and Silva, 1998), whereas inorganic crystals use directly the absorption band of the emitter Ln3+ ion (activator) or ion–ion energy transfer from another codo- pant ion (sensitizer) (Soukka and H€arm€a, 2011).PDF Image | HANDBOOK ON THE PHYSICS AND CHEMISTRY OF RARE EARTHS
PDF Search Title:
HANDBOOK ON THE PHYSICS AND CHEMISTRY OF RARE EARTHSOriginal File Name Searched:
Chemistry-Rare-Earths-49.pdfDIY PDF Search: Google It | Yahoo | Bing
Sulfur Deposition on Carbon Nanofibers using Supercritical CO2 Sulfur Deposition on Carbon Nanofibers using Supercritical CO2. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur... More Info
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |