
PDF Publication Title:
Text from PDF Page: 410
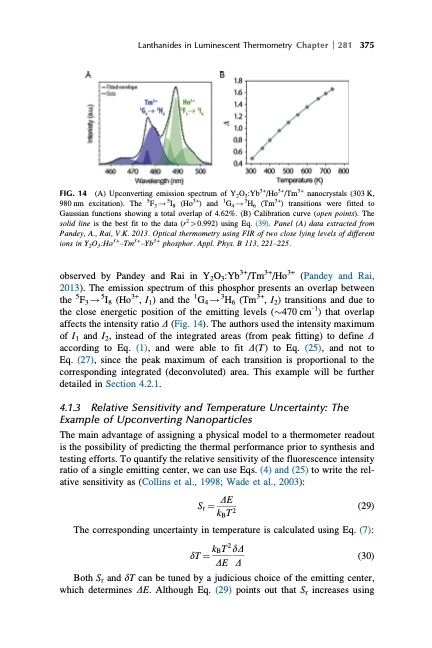
Lanthanides in Luminescent Thermometry Chapter 281 375 FIG. 14 (A) Upconverting emission spectrum of Y2O3:Yb3+/Ho3+/Tm3+ nanocrystals (303 K, 980 nm excitation). The 5F3 ! 5I8 (Ho3+) and 1G4 ! 3H6 (Tm3+) transitions were fitted to Gaussian functions showing a total overlap of 4.62%. (B) Calibration curve (open points). The solid line is the best fit to the data (r2>0.992) using Eq. (39). Panel (A) data extracted from Pandey, A., Rai, V.K. 2013. Optical thermometry using FIR of two close lying levels of different ions in Y2O3:Ho3+–Tm3+–Yb3+ phosphor. Appl. Phys. B 113, 221–225. observed by Pandey and Rai in Y2O3:Yb3+/Tm3+/Ho3+ (Pandey and Rai, 2013). The emission spectrum of this phosphor presents an overlap between the 5F3!5I8 (Ho3+, I1) and the 1G4!3H6 (Tm3+, I2) transitions and due to the close energetic position of the emitting levels ($470 cm˗1) that overlap affects the intensity ratio D (Fig. 14). The authors used the intensity maximum of I1 and I2, instead of the integrated areas (from peak fitting) to define D according to Eq. (1), and were able to fit D(T) to Eq. (25), and not to Eq. (27), since the peak maximum of each transition is proportional to the corresponding integrated (deconvoluted) area. This example will be further detailed in Section 4.2.1. 4.1.3 Relative Sensitivity and Temperature Uncertainty: The Example of Upconverting Nanoparticles The main advantage of assigning a physical model to a thermometer readout is the possibility of predicting the thermal performance prior to synthesis and testing efforts. To quantify the relative sensitivity of the fluorescence intensity ratio of a single emitting center, we can use Eqs. (4) and (25) to write the rel- ative sensitivity as (Collins et al., 1998; Wade et al., 2003): Sr 1⁄4 DE (29) kBT2 The corresponding uncertainty in temperature is calculated using Eq. (7): dT1⁄4kBT2dD (30) DE D Both Sr and dT can be tuned by a judicious choice of the emitting center, which determines DE. Although Eq. (29) points out that Sr increases usingPDF Image | HANDBOOK ON THE PHYSICS AND CHEMISTRY OF RARE EARTHS
PDF Search Title:
HANDBOOK ON THE PHYSICS AND CHEMISTRY OF RARE EARTHSOriginal File Name Searched:
Chemistry-Rare-Earths-49.pdfDIY PDF Search: Google It | Yahoo | Bing
Sulfur Deposition on Carbon Nanofibers using Supercritical CO2 Sulfur Deposition on Carbon Nanofibers using Supercritical CO2. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur... More Info
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |