
PDF Publication Title:
Text from PDF Page: 002
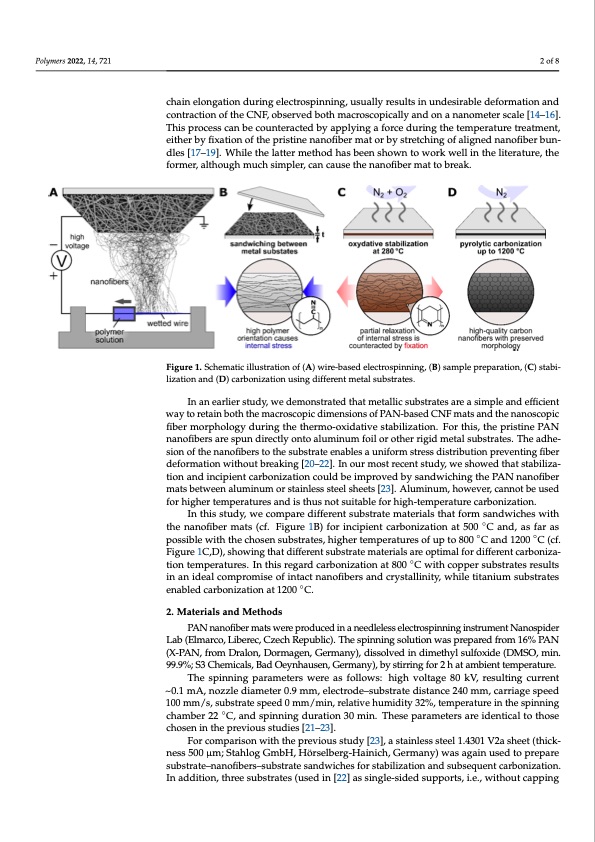
nanoscopic fiber morphology during the thermo‐oxidative stabilization. For this, the pris‐ Polymers 2022, 14, 721 tine PAN nanofibers are spun directly onto aluminum foil or other rigid metal substrates. The adhesion of the nanofibers to the substrate enables a uniform stress distribution pre‐ venting fiber deformation without breaking [20–22]. In our most recent study, we showed that stabilization and incipient carbonization could be improved by sandwiching the PAN nanofiber mats between aluminum or stainless steel sheets [23]. Aluminum, however, can‐ not be used for higher temperatures and is thus not suitable for high‐temperature carbon‐ ization. chain elongation during electrospinning, usually results in undesirable deformation and the nanofiber mats (cf. Figure 1B) for incipient carbonization at 500 °C and, as far as pos‐ contraction of the CNF, observed both macroscopically and on a nanometer scale [14–16]. sible with the chosen substrates, higher temperatures of up to 800 °C and 1200 °C (cf. This process can be counteracted by applying a force during the temperature treatment, Figure 1C and D), showing that different substrate materials are optimal for different car‐ either by fixation of the pristine nanofiber mat or by stretching of aligned nanofiber bun- bonization temperatures. In this regard carbonization at 800 °C with copper substrates dles [17–19]. While the latter method has been shown to work well in the literature, the results in an ideal compromise of intact nanofibers and crystallinity, while titanium sub‐ former, although much simpler, can cause the nanofiber mat to break. strates enabled carbonization at 1200 °C. 2 of 8 In this study, we compare different substrate materials that form sandwiches with Figure 1. Schematic illustration of (A) wire‐based electrospinning, (B) sample preparation, (C) sta‐ Figure 1. Schematic illustration of (A) wire-based electrospinning, (B) sample preparation, (C) stabi- bilization and (D) carbonization using different metal substrates. lization and (D) carbonization using different metal substrates. 2. Materials and Methods In an earlier study, we demonstrated that metallic substrates are a simple and efficient PAN nanofiber mats were produced in a needleless electrospinning instrument Nan‐ way to retain both the macroscopic dimensions of PAN-based CNF mats and the nanoscopic ospider Lab (Elmarco, Liberec, Czech Republic). The spinning solution was prepared from fiber morphology during the thermo-oxidative stabilization. For this, the pristine PAN 16% PAN (X‐PAN, from Dralon, Dormagen, Germany), dissolved in dimethyl sulfoxide nanofibers are spun directly onto aluminum foil or other rigid metal substrates. The adhe- (DMSO, min. 99.9%; S3 Chemicals, Bad Oeynhausen, Germany), by stirring for 2 h at am‐ sion of the nanofibers to the substrate enables a uniform stress distribution preventing fiber bient temperature. deformation without breaking [20–22]. In our most recent study, we showed that stabiliza- The spinning parameters were as follows: high voltage 80 kV, resulting current ~0.1 tion and incipient carbonization could be improved by sandwiching the PAN nanofiber mA, nozzle diameter 0.9 mm, electrode–substrate distance 240 mm, carriage speed 100 mats between aluminum or stainless steel sheets [23]. Aluminum, however, cannot be used mm/s, substrate speed 0 mm/min, relative humidity 32%, temperature in the spinning for higher temperatures and is thus not suitable for high-temperature carbonization. In this study, we compare different substrate materials that form sandwiches with the nanofiber mats (cf. Figure 1B) for incipient carbonization at 500 ◦C and, as far as possible with the chosen substrates, higher temperatures of up to 800 ◦C and 1200 ◦C (cf. Figure 1C,D), showing that different substrate materials are optimal for different carboniza- tion temperatures. In this regard carbonization at 800 ◦C with copper substrates results in an ideal compromise of intact nanofibers and crystallinity, while titanium substrates enabled carbonization at 1200 ◦C. 2. Materials and Methods PAN nanofiber mats were produced in a needleless electrospinning instrument Nanospider Lab (Elmarco, Liberec, Czech Republic). The spinning solution was prepared from 16% PAN (X-PAN, from Dralon, Dormagen, Germany), dissolved in dimethyl sulfoxide (DMSO, min. 99.9%; S3 Chemicals, Bad Oeynhausen, Germany), by stirring for 2 h at ambient temperature. The spinning parameters were as follows: high voltage 80 kV, resulting current ~0.1 mA, nozzle diameter 0.9 mm, electrode–substrate distance 240 mm, carriage speed 100 mm/s, substrate speed 0 mm/min, relative humidity 32%, temperature in the spinning chamber 22 ◦C, and spinning duration 30 min. These parameters are identical to those chosen in the previous studies [21–23]. For comparison with the previous study [23], a stainless steel 1.4301 V2a sheet (thick- ness 500 μm; Stahlog GmbH, Hörselberg-Hainich, Germany) was again used to prepare substrate–nanofibers–substrate sandwiches for stabilization and subsequent carbonization. In addition, three substrates (used in [22] as single-sided supports, i.e., without cappingPDF Image | Carbonization of Electrospun PAN Nanofibers
PDF Search Title:
Carbonization of Electrospun PAN NanofibersOriginal File Name Searched:
polymers-14-00721.pdfDIY PDF Search: Google It | Yahoo | Bing
Sulfur Deposition on Carbon Nanofibers using Supercritical CO2 Sulfur Deposition on Carbon Nanofibers using Supercritical CO2. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur... More Info
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |