
PDF Publication Title:
Text from PDF Page: 221
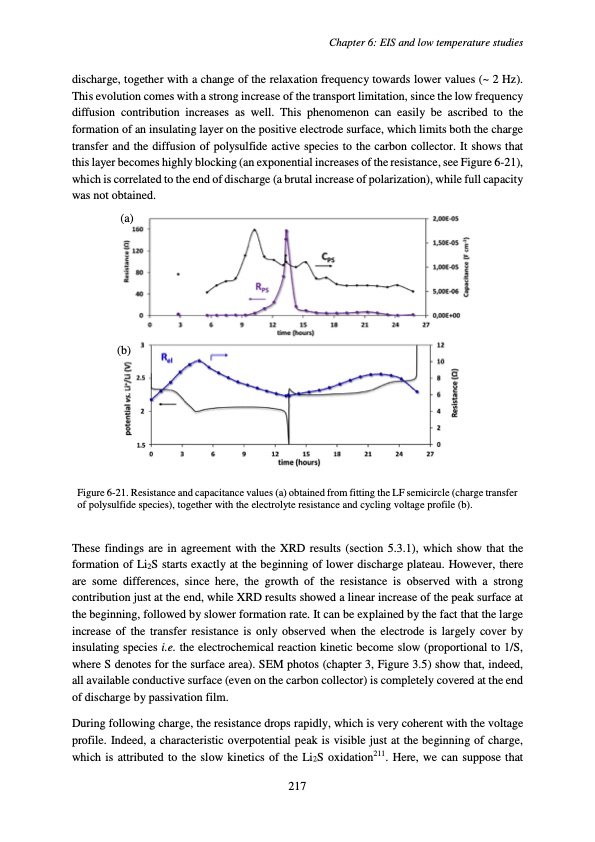
discharge, together with a change of the relaxation frequency towards lower values (~ 2 Hz). This evolution comes with a strong increase of the transport limitation, since the low frequency diffusion contribution increases as well. This phenomenon can easily be ascribed to the formation of an insulating layer on the positive electrode surface, which limits both the charge transfer and the diffusion of polysulfide active species to the carbon collector. It shows that this layer becomes highly blocking (an exponential increases of the resistance, see Figure 6-21), which is correlated to the end of discharge (a brutal increase of polarization), while full capacity was not obtained. (a) (b) Figure 6-21. Resistance and capacitance values (a) obtained from fitting the LF semicircle (charge transfer of polysulfide species), together with the electrolyte resistance and cycling voltage profile (b). These findings are in agreement with the XRD results (section 5.3.1), which show that the formation of Li2S starts exactly at the beginning of lower discharge plateau. However, there are some differences, since here, the growth of the resistance is observed with a strong contribution just at the end, while XRD results showed a linear increase of the peak surface at the beginning, followed by slower formation rate. It can be explained by the fact that the large increase of the transfer resistance is only observed when the electrode is largely cover by insulating species i.e. the electrochemical reaction kinetic become slow (proportional to 1/S, where S denotes for the surface area). SEM photos (chapter 3, Figure 3.5) show that, indeed, all available conductive surface (even on the carbon collector) is completely covered at the end of discharge by passivation film. During following charge, the resistance drops rapidly, which is very coherent with the voltage profile. Indeed, a characteristic overpotential peak is visible just at the beginning of charge, which is attributed to the slow kinetics of the Li2S oxidation211. Here, we can suppose that Chapter 6: EIS and low temperature studies 217PDF Image | Accumulateur Lithium Soufre
PDF Search Title:
Accumulateur Lithium SoufreOriginal File Name Searched:
WALUS_2015_archivage.pdfDIY PDF Search: Google It | Yahoo | Bing
Sulfur Deposition on Carbon Nanofibers using Supercritical CO2 Sulfur Deposition on Carbon Nanofibers using Supercritical CO2. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur... More Info
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |