
PDF Publication Title:
Text from PDF Page: 208
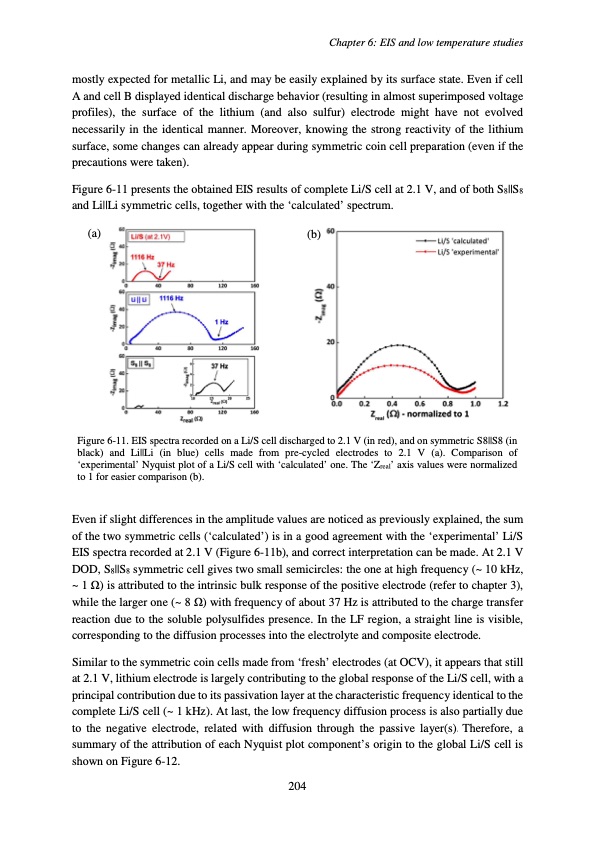
mostly expected for metallic Li, and may be easily explained by its surface state. Even if cell A and cell B displayed identical discharge behavior (resulting in almost superimposed voltage profiles), the surface of the lithium (and also sulfur) electrode might have not evolved necessarily in the identical manner. Moreover, knowing the strong reactivity of the lithium surface, some changes can already appear during symmetric coin cell preparation (even if the precautions were taken). Figure 6-11 presents the obtained EIS results of complete Li/S cell at 2.1 V, and of both S8||S8 and Li||Li symmetric cells, together with the ‘calculated’ spectrum. (a) (b) Chapter 6: EIS and low temperature studies Figure 6-11. EIS spectra recorded on a Li/S cell discharged to 2.1 V (in red), and on symmetric S8||S8 (in black) and Li||Li (in blue) cells made from pre-cycled electrodes to 2.1 V (a). Comparison of ‘experimental’ Nyquist plot of a Li/S cell with ‘calculated’ one. The ‘Zreal’ axis values were normalized to 1 for easier comparison (b). Even if slight differences in the amplitude values are noticed as previously explained, the sum of the two symmetric cells (‘calculated’) is in a good agreement with the ‘experimental’ Li/S EIS spectra recorded at 2.1 V (Figure 6-11b), and correct interpretation can be made. At 2.1 V DOD, S8||S8 symmetric cell gives two small semicircles: the one at high frequency (~ 10 kHz, ~ 1 Ω) is attributed to the intrinsic bulk response of the positive electrode (refer to chapter 3), while the larger one (~ 8 Ω) with frequency of about 37 Hz is attributed to the charge transfer reaction due to the soluble polysulfides presence. In the LF region, a straight line is visible, corresponding to the diffusion processes into the electrolyte and composite electrode. Similar to the symmetric coin cells made from ‘fresh’ electrodes (at OCV), it appears that still at 2.1 V, lithium electrode is largely contributing to the global response of the Li/S cell, with a principal contribution due to its passivation layer at the characteristic frequency identical to the complete Li/S cell (~ 1 kHz). At last, the low frequency diffusion process is also partially due to the negative electrode, related with diffusion through the passive layer(s). Therefore, a summary of the attribution of each Nyquist plot component’s origin to the global Li/S cell is shown on Figure 6-12. 204PDF Image | Accumulateur Lithium Soufre
PDF Search Title:
Accumulateur Lithium SoufreOriginal File Name Searched:
WALUS_2015_archivage.pdfDIY PDF Search: Google It | Yahoo | Bing
Sulfur Deposition on Carbon Nanofibers using Supercritical CO2 Sulfur Deposition on Carbon Nanofibers using Supercritical CO2. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur... More Info
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |