
PDF Publication Title:
Text from PDF Page: 187
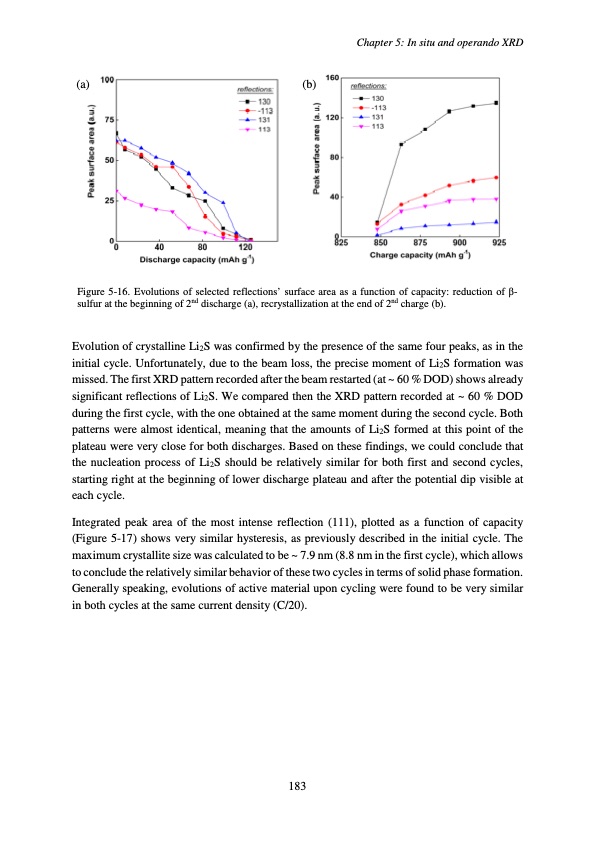
Chapter 5: In situ and operando XRD (a) (b) Figure 5-16. Evolutions of selected reflections’ surface area as a function of capacity: reduction of β- sulfur at the beginning of 2nd discharge (a), recrystallization at the end of 2nd charge (b). Evolution of crystalline Li2S was confirmed by the presence of the same four peaks, as in the initial cycle. Unfortunately, due to the beam loss, the precise moment of Li2S formation was missed. The first XRD pattern recorded after the beam restarted (at ~ 60 % DOD) shows already significant reflections of Li2S. We compared then the XRD pattern recorded at ~ 60 % DOD during the first cycle, with the one obtained at the same moment during the second cycle. Both patterns were almost identical, meaning that the amounts of Li2S formed at this point of the plateau were very close for both discharges. Based on these findings, we could conclude that the nucleation process of Li2S should be relatively similar for both first and second cycles, starting right at the beginning of lower discharge plateau and after the potential dip visible at each cycle. Integrated peak area of the most intense reflection (111), plotted as a function of capacity (Figure 5-17) shows very similar hysteresis, as previously described in the initial cycle. The maximum crystallite size was calculated to be ~ 7.9 nm (8.8 nm in the first cycle), which allows to conclude the relatively similar behavior of these two cycles in terms of solid phase formation. Generally speaking, evolutions of active material upon cycling were found to be very similar in both cycles at the same current density (C/20). 183PDF Image | Accumulateur Lithium Soufre
PDF Search Title:
Accumulateur Lithium SoufreOriginal File Name Searched:
WALUS_2015_archivage.pdfDIY PDF Search: Google It | Yahoo | Bing
Sulfur Deposition on Carbon Nanofibers using Supercritical CO2 Sulfur Deposition on Carbon Nanofibers using Supercritical CO2. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur... More Info
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |