
PDF Publication Title:
Text from PDF Page: 175
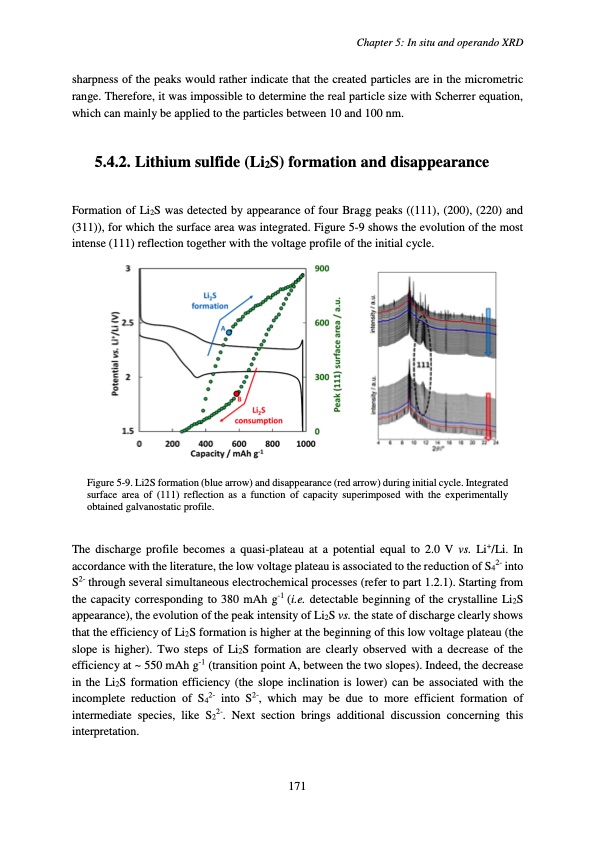
sharpness of the peaks would rather indicate that the created particles are in the micrometric range. Therefore, it was impossible to determine the real particle size with Scherrer equation, which can mainly be applied to the particles between 10 and 100 nm. 5.4.2. Lithium sulfide (Li2S) formation and disappearance Formation of Li2S was detected by appearance of four Bragg peaks ((111), (200), (220) and (311)), for which the surface area was integrated. Figure 5-9 shows the evolution of the most intense (111) reflection together with the voltage profile of the initial cycle. Figure 5-9. Li2S formation (blue arrow) and disappearance (red arrow) during initial cycle. Integrated surface area of (111) reflection as a function of capacity superimposed with the experimentally obtained galvanostatic profile. The discharge profile becomes a quasi-plateau at a potential equal to 2.0 V vs. Li+/Li. In accordance with the literature, the low voltage plateau is associated to the reduction of S42- into S2- through several simultaneous electrochemical processes (refer to part 1.2.1). Starting from the capacity corresponding to 380 mAh g-1 (i.e. detectable beginning of the crystalline Li2S appearance), the evolution of the peak intensity of Li2S vs. the state of discharge clearly shows that the efficiency of Li2S formation is higher at the beginning of this low voltage plateau (the slope is higher). Two steps of Li2S formation are clearly observed with a decrease of the efficiency at ~ 550 mAh g-1 (transition point A, between the two slopes). Indeed, the decrease in the Li2S formation efficiency (the slope inclination is lower) can be associated with the incomplete reduction of S42- into S2-, which may be due to more efficient formation of intermediate species, like S22-. Next section brings additional discussion concerning this interpretation. Chapter 5: In situ and operando XRD 171PDF Image | Accumulateur Lithium Soufre
PDF Search Title:
Accumulateur Lithium SoufreOriginal File Name Searched:
WALUS_2015_archivage.pdfDIY PDF Search: Google It | Yahoo | Bing
Sulfur Deposition on Carbon Nanofibers using Supercritical CO2 Sulfur Deposition on Carbon Nanofibers using Supercritical CO2. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur... More Info
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |