
PDF Publication Title:
Text from PDF Page: 169
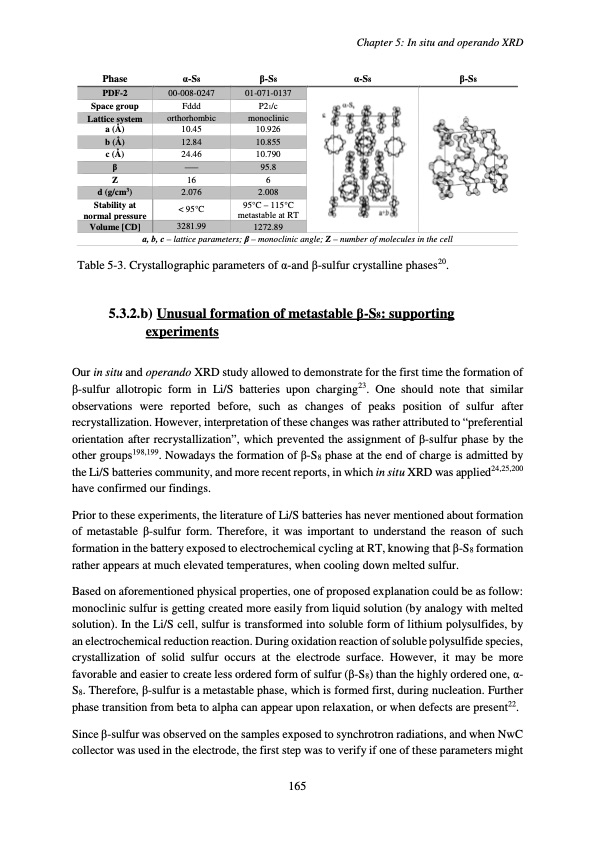
Phase α-S8 β-S8 PDF-2 00-008-0247 01-071-0137 Space group a (Å) Stability at < 95°C 95°C – 115°C normal pressure metastable at RT a, b, c – lattice parameters; β – monoclinic angle; Z – number of molecules in the cell Table 5-3. Crystallographic parameters of α-and β-sulfur crystalline phases20. 5.3.2.b) Unusual formation of metastable β-S8: supporting experiments Our in situ and operando XRD study allowed to demonstrate for the first time the formation of β-sulfur allotropic form in Li/S batteries upon charging23. One should note that similar observations were reported before, such as changes of peaks position of sulfur after recrystallization. However, interpretation of these changes was rather attributed to “preferential orientation after recrystallization”, which prevented the assignment of β-sulfur phase by the other groups198,199. Nowadays the formation of β-S8 phase at the end of charge is admitted by the Li/S batteries community, and more recent reports, in which in situ XRD was applied24,25,200 have confirmed our findings. Prior to these experiments, the literature of Li/S batteries has never mentioned about formation of metastable β-sulfur form. Therefore, it was important to understand the reason of such formation in the battery exposed to electrochemical cycling at RT, knowing that β-S8 formation rather appears at much elevated temperatures, when cooling down melted sulfur. Based on aforementioned physical properties, one of proposed explanation could be as follow: monoclinic sulfur is getting created more easily from liquid solution (by analogy with melted solution). In the Li/S cell, sulfur is transformed into soluble form of lithium polysulfides, by an electrochemical reduction reaction. During oxidation reaction of soluble polysulfide species, crystallization of solid sulfur occurs at the electrode surface. However, it may be more favorable and easier to create less ordered form of sulfur (β-S8) than the highly ordered one, α- S8. Therefore, β-sulfur is a metastable phase, which is formed first, during nucleation. Further phase transition from beta to alpha can appear upon relaxation, or when defects are present22. Since β-sulfur was observed on the samples exposed to synchrotron radiations, and when NwC collector was used in the electrode, the first step was to verify if one of these parameters might P21/c 10.926 24.46 Z 16 6 Fddd 10.45 Chapter 5: In situ and operando XRD Lattice system orthorhombic monoclinic b (Å) 12.84 10.855 c (Å) 10.790 β ––– 95.8 d (g/cm3) 2.076 2.008 Volume [CD] 3281.99 1272.89 165 α-S8 β-S8PDF Image | Accumulateur Lithium Soufre
PDF Search Title:
Accumulateur Lithium SoufreOriginal File Name Searched:
WALUS_2015_archivage.pdfDIY PDF Search: Google It | Yahoo | Bing
Sulfur Deposition on Carbon Nanofibers using Supercritical CO2 Sulfur Deposition on Carbon Nanofibers using Supercritical CO2. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur... More Info
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |