
PDF Publication Title:
Text from PDF Page: 156
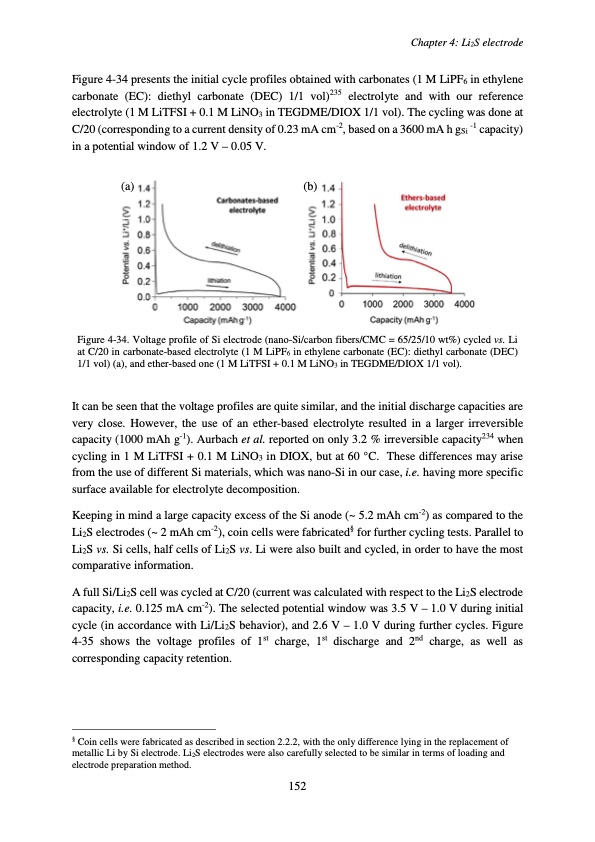
Figure 4-34 presents the initial cycle profiles obtained with carbonates (1 M LiPF6 in ethylene carbonate (EC): diethyl carbonate (DEC) 1/1 vol)235 electrolyte and with our reference electrolyte (1 M LiTFSI + 0.1 M LiNO3 in TEGDME/DIOX 1/1 vol). The cycling was done at C/20 (corresponding to a current density of 0.23 mA cm-2, based on a 3600 mA h gSi -1 capacity) in a potential window of 1.2 V – 0.05 V. (a) (b) Figure 4-34. Voltage profile of Si electrode (nano-Si/carbon fibers/CMC = 65/25/10 wt%) cycled vs. Li at C/20 in carbonate-based electrolyte (1 M LiPF6 in ethylene carbonate (EC): diethyl carbonate (DEC) 1/1 vol) (a), and ether-based one (1 M LiTFSI + 0.1 M LiNO3 in TEGDME/DIOX 1/1 vol). It can be seen that the voltage profiles are quite similar, and the initial discharge capacities are very close. However, the use of an ether-based electrolyte resulted in a larger irreversible capacity (1000 mAh g-1). Aurbach et al. reported on only 3.2 % irreversible capacity234 when cycling in 1 M LiTFSI + 0.1 M LiNO3 in DIOX, but at 60 °C. These differences may arise from the use of different Si materials, which was nano-Si in our case, i.e. having more specific surface available for electrolyte decomposition. Keeping in mind a large capacity excess of the Si anode (~ 5.2 mAh cm-2) as compared to the Li2S electrodes (~ 2 mAh cm-2), coin cells were fabricated§ for further cycling tests. Parallel to Li2S vs. Si cells, half cells of Li2S vs. Li were also built and cycled, in order to have the most comparative information. A full Si/Li2S cell was cycled at C/20 (current was calculated with respect to the Li2S electrode capacity, i.e. 0.125 mA cm-2). The selected potential window was 3.5 V – 1.0 V during initial cycle (in accordance with Li/Li2S behavior), and 2.6 V – 1.0 V during further cycles. Figure 4-35 shows the voltage profiles of 1st charge, 1st discharge and 2nd charge, as well as corresponding capacity retention. § Coin cells were fabricated as described in section 2.2.2, with the only difference lying in the replacement of metallic Li by Si electrode. Li2S electrodes were also carefully selected to be similar in terms of loading and electrode preparation method. Chapter 4: Li2S electrode 152PDF Image | Accumulateur Lithium Soufre
PDF Search Title:
Accumulateur Lithium SoufreOriginal File Name Searched:
WALUS_2015_archivage.pdfDIY PDF Search: Google It | Yahoo | Bing
Sulfur Deposition on Carbon Nanofibers using Supercritical CO2 Sulfur Deposition on Carbon Nanofibers using Supercritical CO2. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur... More Info
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |