
PDF Publication Title:
Text from PDF Page: 146
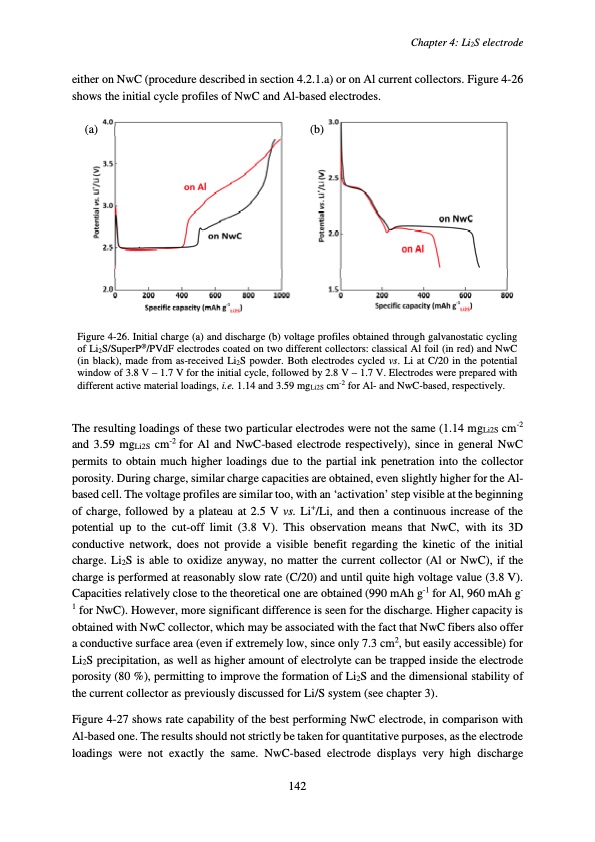
either on NwC (procedure described in section 4.2.1.a) or on Al current collectors. Figure 4-26 shows the initial cycle profiles of NwC and Al-based electrodes. (a) (b) Figure 4-26. Initial charge (a) and discharge (b) voltage profiles obtained through galvanostatic cycling of Li2S/SuperP®/PVdF electrodes coated on two different collectors: classical Al foil (in red) and NwC (in black), made from as-received Li2S powder. Both electrodes cycled vs. Li at C/20 in the potential window of 3.8 V – 1.7 V for the initial cycle, followed by 2.8 V – 1.7 V. Electrodes were prepared with different active material loadings, i.e. 1.14 and 3.59 mgLi2S cm-2 for Al- and NwC-based, respectively. The resulting loadings of these two particular electrodes were not the same (1.14 mgLi2S cm-2 and 3.59 mgLi2S cm-2 for Al and NwC-based electrode respectively), since in general NwC permits to obtain much higher loadings due to the partial ink penetration into the collector porosity. During charge, similar charge capacities are obtained, even slightly higher for the Al- based cell. The voltage profiles are similar too, with an ‘activation’ step visible at the beginning of charge, followed by a plateau at 2.5 V vs. Li+/Li, and then a continuous increase of the potential up to the cut-off limit (3.8 V). This observation means that NwC, with its 3D conductive network, does not provide a visible benefit regarding the kinetic of the initial charge. Li2S is able to oxidize anyway, no matter the current collector (Al or NwC), if the charge is performed at reasonably slow rate (C/20) and until quite high voltage value (3.8 V). Capacities relatively close to the theoretical one are obtained (990 mAh g-1 for Al, 960 mAh g- 1 for NwC). However, more significant difference is seen for the discharge. Higher capacity is obtained with NwC collector, which may be associated with the fact that NwC fibers also offer a conductive surface area (even if extremely low, since only 7.3 cm2, but easily accessible) for Li2S precipitation, as well as higher amount of electrolyte can be trapped inside the electrode porosity (80 %), permitting to improve the formation of Li2S and the dimensional stability of the current collector as previously discussed for Li/S system (see chapter 3). Figure 4-27 shows rate capability of the best performing NwC electrode, in comparison with Al-based one. The results should not strictly be taken for quantitative purposes, as the electrode loadings were not exactly the same. NwC-based electrode displays very high discharge Chapter 4: Li2S electrode 142PDF Image | Accumulateur Lithium Soufre
PDF Search Title:
Accumulateur Lithium SoufreOriginal File Name Searched:
WALUS_2015_archivage.pdfDIY PDF Search: Google It | Yahoo | Bing
Sulfur Deposition on Carbon Nanofibers using Supercritical CO2 Sulfur Deposition on Carbon Nanofibers using Supercritical CO2. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur... More Info
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |