
PDF Publication Title:
Text from PDF Page: 133
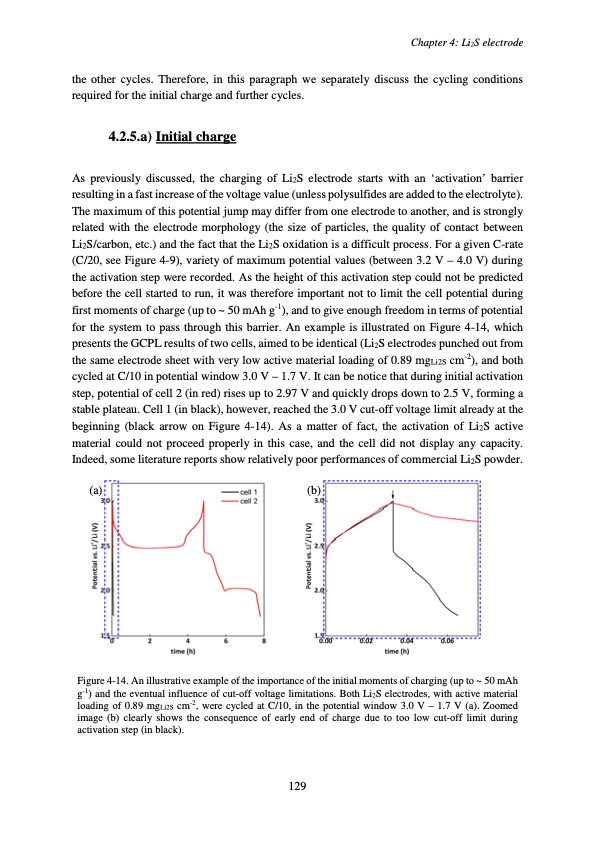
the other cycles. Therefore, in this paragraph we separately discuss the cycling conditions required for the initial charge and further cycles. 4.2.5.a) Initial charge As previously discussed, the charging of Li2S electrode starts with an ‘activation’ barrier resulting in a fast increase of the voltage value (unless polysulfides are added to the electrolyte). The maximum of this potential jump may differ from one electrode to another, and is strongly related with the electrode morphology (the size of particles, the quality of contact between Li2S/carbon, etc.) and the fact that the Li2S oxidation is a difficult process. For a given C-rate (C/20, see Figure 4-9), variety of maximum potential values (between 3.2 V – 4.0 V) during the activation step were recorded. As the height of this activation step could not be predicted before the cell started to run, it was therefore important not to limit the cell potential during first moments of charge (up to ~ 50 mAh g-1), and to give enough freedom in terms of potential for the system to pass through this barrier. An example is illustrated on Figure 4-14, which presents the GCPL results of two cells, aimed to be identical (Li2S electrodes punched out from the same electrode sheet with very low active material loading of 0.89 mgLi2S cm-2), and both cycled at C/10 in potential window 3.0 V – 1.7 V. It can be notice that during initial activation step, potential of cell 2 (in red) rises up to 2.97 V and quickly drops down to 2.5 V, forming a stable plateau. Cell 1 (in black), however, reached the 3.0 V cut-off voltage limit already at the beginning (black arrow on Figure 4-14). As a matter of fact, the activation of Li2S active material could not proceed properly in this case, and the cell did not display any capacity. Indeed, some literature reports show relatively poor performances of commercial Li2S powder. (a) (b) Figure 4-14. An illustrative example of the importance of the initial moments of charging (up to ~ 50 mAh g-1) and the eventual influence of cut-off voltage limitations. Both Li2S electrodes, with active material loading of 0.89 mgLi2S cm-2, were cycled at C/10, in the potential window 3.0 V – 1.7 V (a). Zoomed image (b) clearly shows the consequence of early end of charge due to too low cut-off limit during activation step (in black). Chapter 4: Li2S electrode 129PDF Image | Accumulateur Lithium Soufre
PDF Search Title:
Accumulateur Lithium SoufreOriginal File Name Searched:
WALUS_2015_archivage.pdfDIY PDF Search: Google It | Yahoo | Bing
Sulfur Deposition on Carbon Nanofibers using Supercritical CO2 Sulfur Deposition on Carbon Nanofibers using Supercritical CO2. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur... More Info
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |