
PDF Publication Title:
Text from PDF Page: 131
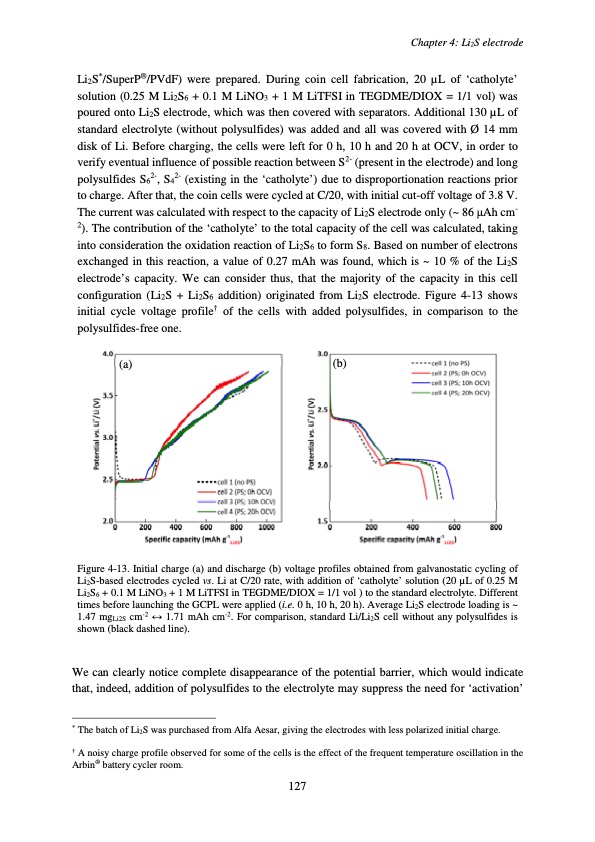
Li2S*/SuperP®/PVdF) were prepared. During coin cell fabrication, 20 μL of ‘catholyte’ solution (0.25 M Li2S6 + 0.1 M LiNO3 + 1 M LiTFSI in TEGDME/DIOX = 1/1 vol) was poured onto Li2S electrode, which was then covered with separators. Additional 130 μL of standard electrolyte (without polysulfides) was added and all was covered with Ø 14 mm disk of Li. Before charging, the cells were left for 0 h, 10 h and 20 h at OCV, in order to verify eventual influence of possible reaction between S2- (present in the electrode) and long polysulfides S62-, S42- (existing in the ‘catholyte’) due to disproportionation reactions prior to charge. After that, the coin cells were cycled at C/20, with initial cut-off voltage of 3.8 V. The current was calculated with respect to the capacity of Li2S electrode only (~ 86 μAh cm- 2). The contribution of the ‘catholyte’ to the total capacity of the cell was calculated, taking into consideration the oxidation reaction of Li2S6 to form S8. Based on number of electrons exchanged in this reaction, a value of 0.27 mAh was found, which is ~ 10 % of the Li2S electrode’s capacity. We can consider thus, that the majority of the capacity in this cell configuration (Li2S + Li2S6 addition) originated from Li2S electrode. Figure 4-13 shows initial cycle voltage profile† of the cells with added polysulfides, in comparison to the polysulfides-free one. (a) (b) Figure 4-13. Initial charge (a) and discharge (b) voltage profiles obtained from galvanostatic cycling of Li2S-based electrodes cycled vs. Li at C/20 rate, with addition of ‘catholyte’ solution (20 μL of 0.25 M Li2S6 + 0.1 M LiNO3 + 1 M LiTFSI in TEGDME/DIOX = 1/1 vol ) to the standard electrolyte. Different times before launching the GCPL were applied (i.e. 0 h, 10 h, 20 h). Average Li2S electrode loading is ~ 1.47 mgLi2S cm-2 ↔ 1.71 mAh cm-2. For comparison, standard Li/Li2S cell without any polysulfides is shown (black dashed line). We can clearly notice complete disappearance of the potential barrier, which would indicate that, indeed, addition of polysulfides to the electrolyte may suppress the need for ‘activation’ * The batch of Li2S was purchased from Alfa Aesar, giving the electrodes with less polarized initial charge. † A noisy charge profile observed for some of the cells is the effect of the frequent temperature oscillation in the Arbin® battery cycler room. Chapter 4: Li2S electrode 127PDF Image | Accumulateur Lithium Soufre
PDF Search Title:
Accumulateur Lithium SoufreOriginal File Name Searched:
WALUS_2015_archivage.pdfDIY PDF Search: Google It | Yahoo | Bing
Sulfur Deposition on Carbon Nanofibers using Supercritical CO2 Sulfur Deposition on Carbon Nanofibers using Supercritical CO2. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur... More Info
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |